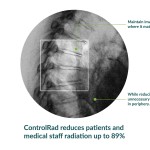
The ControlRad Trace solution reduces unnecessary radiation up to
89%, protecting patients and medical staff without compromising image
quality or workflow.1
ATLANTA–(BUSINESS WIRE)–lt;a href=”https://twitter.com/hashtag/radiationexposure?src=hash” target=”_blank”gt;#radiationexposurelt;/agt;–ControlRad,
Inc., a privately held medical technology company focused on
dramatically reducing unnecessary radiation exposure during
fluoroscopically guided procedures, today announced that the U.S. Food
and Drug Administration (FDA) granted 510(k) clearance for its
ControlRad Trace and the company has initiated its commercial launch.
The ControlRad Trace is the only technology that can be integrated into
existing mobile C-arms to reduce radiation in any fluoroscopic imaging
procedure.
“Radiologists and our teams have grave concerns about the long-term
effects from radiation exposure,” said John A. Carrino, M.D., M.P.H.,
vice chairman of Radiology, Hospital for Special Surgery. “I am excited
that new technology for mobile C-arms is now available because it has
the potential to drastically improve our radiation safety while
maintaining image quality so we can continue to effectively diagnose and
treat our patients. I believe these new products should become the
standard of care for fluoroscopic procedures.”
Fluoroscopically guided procedures (FGP) with C-arms have allowed for
major advances in treating countless diseases, however they expose
patients and medical staff to ionizing radiation, which may increase a
person’s lifetime risk of developing cancer.2 For example, an
interventional fluoroscopy procedure is roughly equivalent to the adult
effective dose of between 250-3,500 chest X-rays.3
“Radiation from C-arm procedures may increase the risk of brain cancer,
cataracts, strokes and atherosclerosis,” says Guillaume Bailliard,
ControlRad chief executive officer. “It’s our mission to dramatically
reduce these life-altering radiation health risks to medical personnel
and patients. This FDA clearance for ControlRad Trace allows us to
provide our valuable radiation reduction technology to all mobile C-arm
users looking to provide a safer environment without compromising
patient care.”
With its proprietary semi-transparent filter, tablet and image
processing technology, the ControlRad Trace solution can be retrofitted
on existing C-arms, reducing the barrier to adopting the technology in
order to reduce unnecessary radiation up to 89%,1 without
compromising image quality in the region of interest and overall
workflow. The medical staff draws a region of interest (ROI) on a
ControlRad tablet, which in real-time optimizes image quality in the ROI
while reducing unnecessary radiation in the periphery.
“With over 17 million fluoroscopic procedures in the U.S. every year,
the ControlRad Trace is the only system available that can impact all of
them,” says Chris Fair, ControlRad executive vice president and
president, Mobile C-arm division. “Regardless if it is a pain management
treatment, an orthopedic trauma case or even a minimally invasive spine
procedure, our technology will reduce radiation exposure and protect
those who are saving the lives of others.”
ControlRad is now initiating the commercialization of ControlRad Trace.
To learn more about ControlRad Trace, visit www.ControlRad.com
or email info@ControlRad.com.
ControlRad is a privately held medical technology company developing
innovative products that dramatically reduce the radiation exposure from
fluoroscopically guided procedures (FGP) for patients and healthcare
professionals. ControlRad’s products are designed to improve safety
without compromising image quality or workflow. They include an
integrated set of proprietary components, which optimize the X-ray beam
to deliver optimal image quality in the clinically relevant region while
maintaining appropriate resolution in the periphery. ControlRad is
headquartered in Atlanta, Georgia, and has engineering development
facilities in Kfar Saba, Israel. For more information, visit www.controlrad.com.
1 Data on file
2https://www.fda.gov/radiation-emitting-products/initiative-reduce-unnecessary-radiation-exposure-medical-imaging/white-paper-initiative-reduce-unnecessary-radiation-exposure-medical-imaging,
p. 1
3 Ibid, p. 3
Contacts
Joyce Ip
Merryman Communications
Joyce@MerrymanCommunications.com
760-889-0438
ARLINGTON, Va., June 20, 2025 /PRNewswire/ -- New survey data from 408 global scientists and researchers…
WAYNE, N.J., June 20, 2025 /PRNewswire/ -- CONSTANT MEDIA LLC, a leader in Point of…
This Fourth of July, Dr. Harbpinder Shevchenko, DMD of Smiles of Salem is going beyond…
Pittsburgh Event Celebrates Employees, Partners and a Commitment to Growth DUQUESNE, Pa., June 20, 2025…
SEAFORD, Del., June 20, 2025 /PRNewswire/ -- Acclaimed physician, entrepreneur, and visionary healthcare leader Nihar Gala…
Toronto, Ontario--(Newsfile Corp. - June 20, 2025) - LevelJump Healthcare Corp. (TSXV: JUMP) ("LevelJump" or the…