Theralase Releases 1Q 2019 Financial Statements
TORONTO, ON / ACCESSWIRE / May 30, 2019 / Theralase® Technologies Inc. (“Theralase” or the “Company“) (TSXV: TLT) (OTCQB: TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds (“PDC“) and their associated drug formulations intended to safely and effectively destroy various cancers released its 1Q2019 financial statements.
Total revenue for the three-month period ended March 31, 2019, decreased to $121,179 from $441,193 for the same period in 2018, a 73% decrease. In Canada, revenue decreased 61% to $116,104 from $297,061. In the US, revenue decreased 94% to $5,075 from $90,354 and international revenue decreased 100% from $53,778 to $Nil during the three-month period ended March 31, 2019. The decrease in total revenue in 2019 is primarily due to the restructuring of the sales and marketing departments resulting in the termination of certain sales and marketing personnel in 2018 and the hiring of new marketing personnel in March 2019.
Cost of sales for the three-month period ended March 31, 2019 was $84,251 (70% of revenue) resulting in a gross margin of $36,928 or 30% of revenue, compared to a cost of sales of $242,857 (55% of revenue) in 2018, resulting in a gross margin of $198,336 or 45% of revenue. Cost of sales is represented by the following costs: raw materials, subcontracting, direct and indirect labour and the applicable share of manufacturing overhead.
The gross margin as a percentage of sales decrease, year over year, is attributed primarily to decreased sales and fixed production salaries for the TLC-1000 and TLC-2000 product lines.
For the three-month period ended March 31, 2019, sales and marketing expenses decreased to $178,807 or 148% of sales, from $280,874 or 64% of sales in 2018, a 36% decrease.
The decrease in sales and marketing expenses is primarily due to the restructuring of the Canadian and US sales and marketing departments, resulting in the termination of certain sales and marketing personnel in 2018 and the hiring of new marketing personnel in March 2019.
Administrative expenses for the three-month period ended March 31, 2019 decreased to $533,659 from $555,086 in 2018, representing a 4% decrease.
Decreases in administrative expenses are attributed to the following:
- Administrative salaries decreased by 10% due to the termination and/or resignation of certain administrative staff.
- Professional fees decreased 45% due to reduced spending on legal fees as a result of the OSC Settlement Agreement.
Net research and development expenses for the three-month period ended March 31, 2019, increased to $447,751 from $364,956 in 2018, a 23% increase.
Research and development expenses for the three-month period ended March 31, 2019, increased primarily due to increased expenses for commencing the Anti-Cancer Technology – Non-Muscle Invasive Bladder Cancer (“ACT NMIBC“) Phase II Study.
As of March 25, 2019, the Company has recommenced development of the next generation TLC-2000 laser system with an expected completion in 2H2019.
Research and development expenses represented 39% of the Company’s operating expenses for the three-month period ended March 31, 2019 and represent investment into the research and development of the Company’s Anti-Cancer Therapy (“ACT“).
The net loss for the three-month period ended March 31, 2019 was $1,125,098, which included $93,756 of net non-cash expenses (i.e.: amortization, stock-based compensation expense, foreign exchange gain/loss and lease inducements). This compared to a net loss for the same period in 2018 of $1,004,068, an increase of 12%, which included $36,405 of net non-cash expenses.
The ACT division represented $506,976 of this loss (45%) for the three-month period ended March 31, 2019.
The increase in net loss is primarily attributed to:
- Increased investment in research and development in the ACT NMIBC Phase II clinical study.
- Decreased sales of the TLC-1000 and TLC-2000.
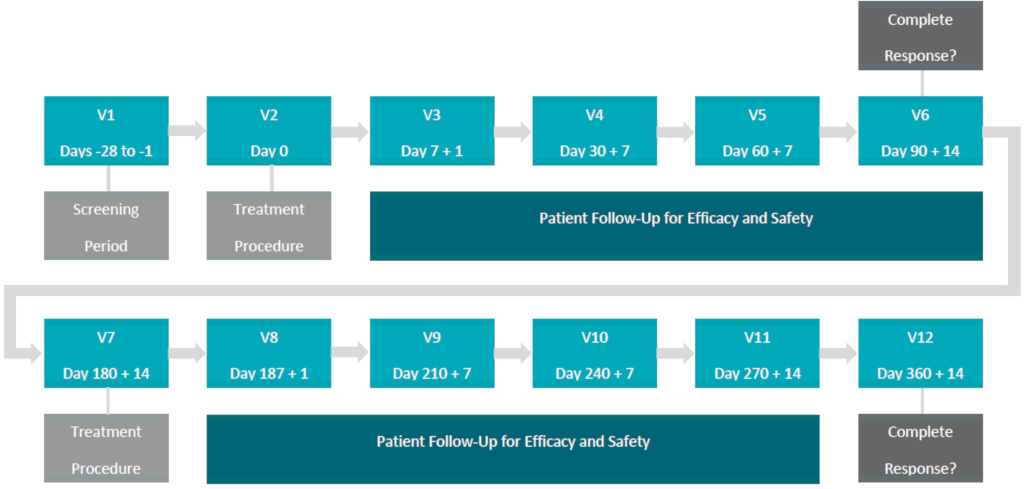
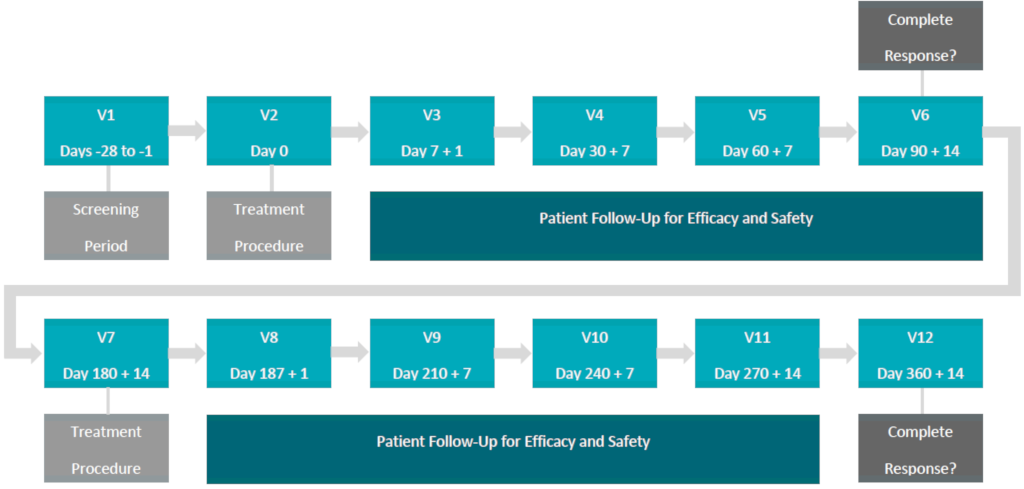
In the Phase Ib NMIBC Clinical study (“Study“), the primary endpoint (safety and tolerability), secondary endpoint (pharmacokinetics) and exploratory endpoint (efficacy) was demonstrated in 2 out of 3 patients (66% Complete Response (“CR“)) treated at the Therapeutic Dose (0.70 mg/cm2) and confirmed by the Medical and Scientific Advisory Board (“MSAB“) on May 19, 2018.
As a result, the MSAB voted to unanimously terminate the Study early and commence the design of a multi-center Phase II NMIBC clinical study (“Study II“) with a primary endpoint of efficacy to be conducted in Canada, the United States and internationally, subject to Health Canada, FDA and international regulatory approval.
The primary endpoint of efficacy will be determined by Complete Response (“CR“) and duration of CR in approximately 100 patients, who present with Carcinoma In-Situ (“CIS“), with or without resected papillary disease (Ta, T1) high grade, are considered BCG-Unresponsive and meet the inclusion / exclusion criteria of Study II.
Health Canada issued a No Objection Letter in response to the Company’s Clinical Trial Application (“CTA“) for its lead PDC, TLD-1433 (November 2018) and an approval for the Company’s Investigational Testing Authorization (“ITA“) for its TLC-3200 Medical Laser System (December 2018). The CTA and ITA approval allowed the Company to commence Study II, subject to submitting a Clinical Trial Site Information Form and receipt of a Research Ethics Board (“REB“) approval for each Canadian oncology location that will conduct Study II.
University Health Hospital (“UHN“) is the first site to obtain REB approval and commence Study II and is currently screening patients for enrollment in Study II.
The Company is currently working with 3 additional institutional clinical oncology sites, already in the final negotiation phases with the Company, for commencing Study II in their respective organization in 3Q 2019.
Theralase has confirmed a conference call with the Food and Drug Administration (“FDA“) to discuss the design and endpoints of Study II, via a Pre-Investigational New Drug (“PIND“) meeting in late June 2019. Pending completion of this conference call, the Company plans to submit an Investigational New Drug (“IND“) application to the FDA and subject to their final review and approval, the Company will commence on-boarding institutional clinical oncology sites in the United States for Study II.
About Phase II Study
The ACT-NMIBC clinical study will utilize the Therapeutic Dose (0.70 mg/cm2) of TLD-1433 and will focus on the treatment of approximately 100 BCG-Unresponsive NMIBC patients in approximately 20 clinical study sites located in Canada, the US and internationally, with a primary endpoint of efficacy and a secondary endpoint of safety.
The primary endpoint will be evaluated by:
CR in patients with Carcinoma In-Situ (“CIS“) with or without resected papillary disease at 90 days post-treatment with a duration of CR evaluated at 360 days post-treatment.
Patient CR is defined as at least one of the following:
1) Negative cystoscopy and negative (including atypical) urine cytology
2) Positive cystoscopy with biopsy-proven benign or low-grade NMIBC
3) Negative cystoscopy with malignant urine cytology, if cancer is found in the upper tract or prostatic urethra and random bladder biopsies are negative
The secondary endpoint will be evaluated by:
Incidence and severity of Adverse Events (“AEs“) Grade 4 or higher that do not resolve within 360 days post-treatment; whereby:
Grade 1 = Mild
Grade 2 = Moderate
Grade 3 = Severe
Grade 4 = Life-threatening or disabling
Grade 5 = Death
Proposed Clinical Treatment Plan:
About Theralase® Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds and their associated drug formulations intended to safely and effectively destroy various cancers.
Additional information is available at www.theralase.com and www.sedar.com
This news release contains “forward-looking statements” which reflect the current expectations of management of the Company’s future growth, results of operations, performance and business prospects and opportunities. Such statements include, but are not limited to, statements regarding the Company’s proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Wherever possible, words such as “may“, “would“, “could“, “should“, “will“, “anticipate“, “believe“, “plan“, “expect“, “intend“, “estimate“, “potential for” and similar expressions have been used to identify these forward-looking statements. These statements reflect management’s current beliefs with respect to future events and are based on information currently available to management. Forward-looking statements involve significant risks, uncertainties and assumptions including with respect to the ability of the Company to: adequately fund, secure the requisite regulatory approvals to commence and successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its development plans. Many factors could cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements; including, without limitation, those listed in the filings made by the Company with the Canadian securities regulatory authorities (which may be viewed at www.sedar.com). Should one or more of these risks or uncertainties materialize or should assumptions underlying the forward looking statements prove incorrect, actual results, performance or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. The Company disclaims any intention or obligation to revise forward-looking statements whether as a result of new information, future developments or otherwise except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchanges) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
1.866.THE.LASE (843-5273) x 304
416.699.LASE (5273) x 304
Amelia Tudo, Investor Relations Coordinator
atudo@theralase.com
www.theralase.com
SOURCE: Theralase Technologies Inc.
View source version on accesswire.com:
https://www.accesswire.com/547239/Theralase-Releases-1Q-2019-Financial-Statements
