Motus GI Receives FDA Clearance to Market Pure-Vu® GEN2
– FDA clearance marks key regulatory milestone and advances
commercial strategy for launch in U.S. hospital market in 2019 –
FORT LAUDERDALE, Fla.–(BUSINESS WIRE)–Motus
GI Holdings, Inc., (NASDAQ: MOTS) (“Motus GI” or the “Company”), a
medical technology company dedicated to improving clinical outcomes and
enhancing the cost-efficiency of colonoscopy, today announced it has
received 510(k) clearance from the U.S. Food and Drug Administration
(“FDA”) for the second-generation Pure-Vu®
System (“Pure-Vu® GEN2”) to help facilitate the cleaning of a poorly
prepared colon during the colonoscopy procedure.
“Inadequate bowel preparation prior to colonoscopy remains an unmet need
that affects a significant percentage of patients’ ability to receive a
complete and high-quality exam. This often leads to canceled, delayed
and aborted procedures, resulting in prolonged hospitalizations and
increased costs for both patients and providers,” commented Jason B.
Samarasena, MD FACG, Associate Clinical Professor of Medicine, Division
of Gastroenterology School of Medicine, University of California Irvine.
“The Pure-Vu® System provides an important solution to address the
significant clinical challenges and inefficiencies associated with
inadequate prep and the Pure-Vu® GEN2 provides an innovative, easy to
use platform that enables a streamlined approach to the overall
procedure.”
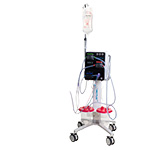
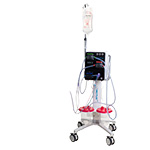
The Pure-Vu® System is a U.S. FDA-cleared medical device indicated to
help facilitate the cleaning of a poorly prepared colon during the
colonoscopy procedure. The device integrates with standard and slim
colonoscopes to enable safe and rapid cleansing during the procedure
while preserving established procedural workflow and techniques.
Pure-Vu® GEN2 has been designed to improve the mobility, setup logistics
of the system and enhance navigation through the colon, while retaining
all the same cleansing functionality as the first generation of the
Pure-Vu® System.
“Receiving FDA clearance for our Pure-Vu® GEN2 represents a major
milestone for the Company. The Pure-Vu® System continues to demonstrate
outstanding cleansing performance in poorly prepped colons, including
the statistically significant improvement in colon cleanliness in
hospitalized patients as recently demonstrated by the positive outcome
of our REDUCE study. With our robust portfolio of health economic and
clinical data coupled with 510(k) clearance from the FDA of Pure-Vu®
GEN2, we are now well positioned to execute our planned commercial
launch of the Pure-Vu® System this year and advance toward our goal of
establishing the Pure-Vu® System as a new standard of care in key
endoscopy segments,” commented Tim
Moran, Chief Executive Officer of Motus GI.
Our planned initial launch will focus on the inpatient colonoscopy
market where challenges with insufficient bowel prep slow diagnosis,
diminish the quality of care, and add significant costs to hospital
systems. Motus GI believes that the Pure-Vu® System may improve quality
of care and potentially reduce healthcare costs by reliably and
predictably moving patients through the hospital system to a successful
examination.
About Motus GI and the Pure-Vu® System
Motus GI Holdings, Inc. is a medical technology company, with
subsidiaries in the U.S. and Israel, dedicated to improving clinical
outcomes and enhancing the cost-efficiency of colonoscopy. The Company’s
flagship product is the Pure-Vu® System, a U.S. FDA cleared medical
device indicated to help facilitate the cleaning of a poorly prepared
colon during the colonoscopy procedure. The device integrates with
standard and slim colonoscopes to enable safe and rapid cleansing during
the procedure while preserving established procedural workflow and
techniques. The Pure-Vu® System has received CE mark approval in Europe.
The Pure-Vu® System is currently being introduced on a pilot basis in
the U.S. market, and the Company is planning to initiate a commercial
launch focused on the U.S. hospital market in 2019. Challenges with
bowel preparation for inpatient colonoscopy represent a significant area
of unmet need that directly affects clinical outcomes and increases the
cost of care in a market segment that comprises approximately 1.5
million annual procedures in the U.S. and approximately 4 million annual
procedures worldwide. Motus GI believes the Pure-Vu® System may improve
outcomes and lower costs for hospitals by reducing the time to
successful colonoscopy, minimizing delayed and incomplete procedures,
and improving the quality of an exam. In clinical studies to date, the
Pure-Vu® System significantly increased the number of patients with an
adequate cleansing level, according to the Boston Bowel Preparation
Scale Score, a validated assessment instrument.
For more information, visit www.motusgi.com
and connect with the Company on Twitter,
LinkedIn
and Facebook.
Forward-Looking Statements
This press release contains certain forward-looking statements.
Forward-looking statements are based on the Company’s current
expectations and assumptions. The Private Securities Litigation Reform
Act of 1995 provides a safe-harbor for forward-looking statements. These
statements may be identified by the use of forward-looking expressions,
including, but not limited to, “expect,” “anticipate,” “intend,” “plan,”
“believe,” “estimate,” “potential,” “predict,” “project,” “should,”
“would” and similar expressions and the negatives of those terms,
including without limitation, risks inherent in the development and
commercialization of potential products, uncertainty in the timing and
results of clinical trials or regulatory approvals, maintenance of
intellectual property rights or other risks discussed in the Company’s
Form 10-K filed on March 26, 2019, and its other filings with the
Securities and Exchange Commission. Prospective investors are cautioned
not to place undue reliance on such forward-looking statements, which
speak only as of the date hereof. The Company undertakes no obligation
to publicly update any forward-looking statement, whether as a result of
new information, future events or otherwise.
Contacts
Media:
Erich A. Sandoval
Lazar
Partners
(917) 497-2867
esandoval@lazarpartners.com
Investors:
Jenene Thomas
Jenene
Thomas Communications, LLC
(833) 475-8247
mots@jtcir.com