Murata Vios, Inc. Announces FDA Clearance for Second Generation Wireless Monitoring Platform
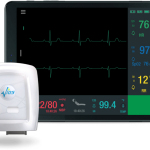
Company preparing to introduce an advanced continuous patient
monitoring solution allowing for cost-effective management of patients
throughout the care continuum
ST. PAUL, Minn.–(BUSINESS WIRE)–Murata Vios, Inc., a medical technology company that develops wireless
patient-monitoring sensors and software, announced that it has received
U.S. Food and Drug Administration (FDA) clearance for two 510(k)
submissions for its second-generation wireless patient monitoring
platform, the Vios Monitoring System (VMS). The approved suite of
products provides a platform designed to reduce the cost of
patient-monitoring equipment while simultaneously striving to improve
the quality of care and the patient experience.
The second-generation platform collects 7-leads of ECG, heart rate,
respiratory rate, SpO2, blood pressure, temperature, pulse
rate, patient posture and activity data to conveniently and
cost-effectively monitor patients throughout various clinical
environments. Murata Vios, Inc. is currently working with hospitals and
post-acute care facilities as it prepares for its U.S. commercial launch.
The VMS is designed to optimize the monitoring of patients throughout
the healthcare facility by providing a lower cost, user-friendly, and
secure patient monitoring platform. One of the significant advantages is
that Vios can continuously monitor and display patient vital signs
across a multitude of devices utilizing sensors, unique signal
processing IP, and propriety software that runs on standard
off-the-shelf hardware. Based on the low-bandwidth connected data,
healthcare providers will be able to remotely monitor patient data in
near real-time. This design strategy significantly reduces the cost of
the overall solution and removes the enormous dependence on expensive
proprietary IT networking equipment.
“Our FDA clearance is a big step in our commitment to patient safety and
providing a premium patient experience. The U.S. healthcare landscape is
rapidly evolving, and we look forward to providing efficient and secure
solutions that help manage patients throughout the care continuum,” said
Varun Verma, Senior Manager at Murata Vios.
“Our team has worked long and hard to achieve this milestone and we are
excited to finally bring our solution to market. Now that we have been
acquired by Murata Manufacturing of Japan, we will be able to support
and scale our solution in ways that very few innovative technologies
can,” said Amit Patel, Chief Executive Officer of Murata Vios.
For more information about the Vios Monitoring System or to connect with
the Murata Vios team, please visit www.viosmedical.com.
About Murata Vios, Inc.
Murata Vios, Inc., a subsidiary of Murata Manufacturing Co., Ltd., is a
medical technology company that delivers user-friendly, cost-effective
and more efficient wireless patient monitoring technologies through a
medical-grade sensor and software-based Internet of things (IoT)
framework. Using sensors that monitor patient vital signs such as heart
and respiratory rates in conjunction with proprietary software that runs
on standard mobile devices and computers, health care providers can
effectively monitor patients at lower cost and greater convenience to
clinicians and patients. Murata Vios, Inc. was formerly known as Vios
Medical, Inc. prior to its acquisition by Murata Manufacturing in
October 2017.
Murata Manufacturing Co., Ltd. is a worldwide leader in the design,
manufacture and sale of ceramic-based passive electronic components &
solutions, communication modules and power supply modules. Murata is
committed to the development of advanced electronic materials and
leading edge, multi-functional, high-density modules. The company has
employees and manufacturing facilities throughout the world. For more
information, visit Murata’s website at www.murata.com.
Contacts
Varun Verma
varun.verma.@murata.com

