Boston Scientific Announces FDA Approval Of ImageReady™ MRI For Vercise Gevia™ Deep Brain Stimulation System
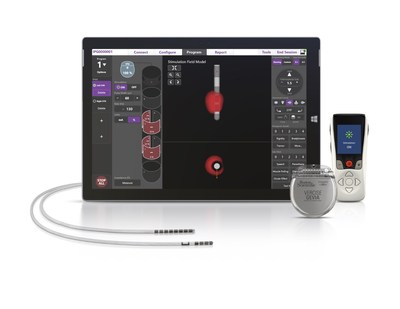
MARLBOROUGH, Mass., Aug. 19, 2019 /PRNewswire/ — Boston Scientific Corporation (NYSE:BSX) announced the U.S. Food and Drug Administration (FDA) approval of its ImageReady™ MRI labeling for the Vercise Gevia™ Deep Brain Stimulation (DBS) System to be used in a full-body magnetic resonance imaging (MRI) environment.1 This system, with the Vercise Cartesia™ Directional Lead, is designed to treat the symptoms of Parkinson’s Disease (PD) by delivering precisely targeted electrical stimulation in the brain to provide optimal symptom relief and better control of unwanted side effects.

An estimated ten million people worldwide are affected by Parkinson’s disease, causing symptoms such as shaking or tremors, muscle stiffness, and slowness of movement.2 DBS therapy helps patients with Parkinson’s disease control their symptoms and improve their quality of life. This FDA approval allows patients to undergo a full-body MRI1 while benefiting from the latest advances in DBS therapy including directional stimulation and a longer-lasting rechargeable battery.
“When evaluating which DBS system is best for each of my patients, I always consider the immediate and long term needs my patient might have so that we can effectively address a patient’s therapeutic needs even as their disease progresses,” said Dr. Robert Gross, MBNA Bowman Endowed Chair in Neurosurgery & Professor Department of Neurosurgery at Emory University. “Customizable therapy, battery life, the size of the device, and access to MRI are factors patients should talk to their doctor about when they are considering deep brain stimulation.”
Clinical evidence from the INTREPID study demonstrated that patients treated with the Vercise System sustained a 48% improvement in motor function as measured by the Unified Parkinson’s Disease Rating Scale (UPDRS) III scores over two years3, which is favorable to previous published reports (25%, Follett, 2010).4
“Boston Scientific continually strives to deliver new solutions that advance the field of neuromodulation and most importantly, result in better outcomes for our patients around the world,” said Maulik Nanavaty, senior vice president and president, Neuromodulation, Boston Scientific.
The Vercise Gevia with Cartesia Directional Lead was approved by the U.S. FDA in January 2019, following the first-generation Vercise DBS System approval in December 2017. The ImageReady™ MRI Vercise Gevia System has also been commercially available in the European market since 2017.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 35 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
1 1.5 Tesla MRI conditional when all conditions of use are met.
2 http://parkinson.org/Understanding-Parkinsons/Causes-and-Statistics/Statistics
3Vitek, J., et. al. A Two-Year Follow-Up of a Prospective, Double Blinded, Multi-Center Randomized Controlled Trial Evaluating Deep Brain Stimulation with a New Multiple Source, Constant-Current Rechargeable System for Treatment of Parkinson’s Disease (INTREPID). INS 2019, Sydney, Australia.
4Follett et al., N EnglJ Med. 2010 Jun 3;362(22):2077-91. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like “anticipate,” “expect,” “project,” “believe,” “plan,” “estimate,” “intend” and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our product launches and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; the closing and integration of acquisitions; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Rochelle Silsbee
Media Relations
(818) 812-7320 (office)
Rochelle.Silsbee@bsci.com
Susie Lisa, CFA
Investor Relations
(508) 683-5565 (office)
BSXInvestorRelations@bsci.com
View original content to download multimedia:http://www.prnewswire.com/news-releases/boston-scientific-announces-fda-approval-of-imageready-mri-for-vercise-gevia-deep-brain-stimulation-system-300903892.html
SOURCE Boston Scientific Corporation
