Path-breaking Study Reports High Sensitivity in Diabetic Retinopathy Screening Using Smartphones and Automated-AI in Primary-Care: Remidio Innovative Solutions
BENGALURU, India, Oct. 10, 2019 /PRNewswire/ — Nearly 50% of patients with diabetes fail to get an annual eye examination done even in the US and EU. In many countries, less than 10% of patients referred to ophthalmologists by their primary-care physicians actually complete a dilated retinal examination.

Technology can play a role in empowering primary-care physicians. Primary-care based Diabetic Retinopathy (DR) screening can enable early detection, avoiding blindness due to DR for close to 425 million people living with diabetes globally.
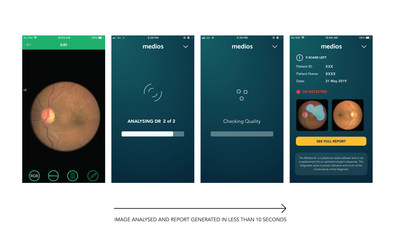
In a study reported in JAMA Ophthalmology, a team from Aditya Jyot Foundation for Twinkling Little Eyes, Mumbai, India, screened patients with diabetes mellitus in civic dispensaries in Mumbai with smartphone-based retinal cameras from Remidio Innovative Solutions and validated the diagnostic accuracy of the integrated Medios AI to detect DR. Medios AI provides a retinal diagnosis report in less than 10 seconds, with inferencing done right on the smartphone.
The images captured on Remidio’s smartphone camera were subjected to automated analysis by Medios AI. They were simultaneously graded by vitreoretinal specialists. The Medios AI is composed of two different algorithms based on convolutional neural networks, one assessing the image-quality, and the other separating healthy images from images with referable DR (RDR).
The clinical sensitivity and specificity of the Medios AI algorithm were found to be 100% and 88.4% for RDR, 85.2% and 92.0% for any DR respectively, exceeding FDA’s superiority end points of an RDR sensitivity of 85% and specificity of 82.5%.
Dr. T.Y. Alvin Liu, MD, Assistant Professor, Ophthalmology at Johns Hopkins Bloomberg School of Public Health, in an invited commentary, calls the study “paradigm-shifting”.
Dr. Natarajan, the lead author of the study, opines, “The study paves the way in implementing large-scale models for screening for DR, even in locations with no Internet infrastructure. This is a step ahead of the approach taken by Google and other AI companies that need their AI models to run on external servers, necessitating Internet access.”
The device and automated AI are CE marked and available for use in the EU. Previous clinical studies validating the same device were published in Nature Eye and Ophthalmology Retina.
About Remidio Innovative Solutions:
Remidio is the world’s first integrated ophthalmic device and Artificial Intelligence company that uses its patented and easy-to-use products to impact vision screening, globally. For more information, visit: http://www.remidio.com/
Media contact
Alok Raj K
+91-7353539000
alokraj@remidio.com
SOURCE Remidio Innovative Solutions Pvt. Ltd.
