Signant Health Partners with Propeller Health to Gather Accurate Data on Asthma and COPD Medication Use
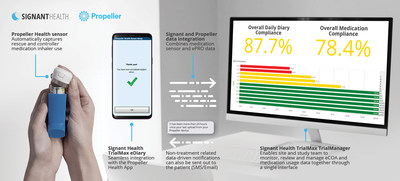
PHILADELPHIA, Jan. 7, 2020 /PRNewswire/ — Signant Health announced today a partnership to connect TrialMax®, the industry-leading electronic Clinical Outcome Assessment (eCOA) platform, to Propeller Health’s digital health platform for asthma and COPD (chronic obstructive pulmonary disease).

The partnership will help drive patient adherence to inhaled medications by enabling accurate time tracking of patients’ medication use events alongside their eDiary data within a single eCOA platform. Sponsors, CROs, and academic institutions can use this information to identify how patients are following treatment plans and identify occurrences when symptoms are triggered, leading to better inhaler adherence, symptom control, and a simpler patient experience.
As always, TrialMax users benefit from a streamlined workflow presented intuitively in a single workspace. Sites and sponsors can view and report on medication use alongside clinical outcomes while study participants experience a simple and burden-free trial, as the Propeller sensors automatically track rescue and controller medication inhaler use. This level of insight allows researchers to track how populations are using inhalers, while still providing valuable insight to oversee the progress and care of individual patients as needed.
Bill Byrom, Signant Health’s VP Product Strategy & Innovation, said, “This integration is important in understanding inhaler use and patterns in patients with respiratory illnesses. This will enable researchers to relate medication usage to responses to treatment, and for site personnel to gain deeper insights together with eCOA data to enhance patient oversight between clinic visits, in addition to encouraging medication adherence where appropriate. The addition of the Propeller sensor within TrialMax’s ecosystem of connected wearables and sensors provides a frictionless way to collect medication usage data without requiring additional actions by the patient. The Propeller Health partnership is an important addition to our solutions for patient-facing technology to enhance data collection and insights in clinical trials in respiratory illnesses.”
About Signant Health
The best technology succeeds in the background. Signant Health provides solutions that simplify every step of the study participant journey to make it easier for people to participate in, and for sites and study teams to run, clinical trials. Signant unites eCOA, eConsent, Patient Engagement, IRT, Clinical Supplies and Endpoint Quality into the industry’s most comprehensive patient-centric suite – an evolution built on more than 20 years of proven clinical research technology. Our intense focus on the patient experience, deep therapeutic area expertise and global operational scale enable hundreds of sponsors and CROs (including all Top 20 pharma) to extend the reach of drug development, expand patient opportunities and improve data quality – helping them bring life-changing therapies to our families and communities around the world. Take a significant step toward patient-centricity at signanthealth.com.
Contact: media@signanthealth.com, +1-267-498-2350
https://www.linkedin.com/company/signant-health/
https://www.facebook.com/signanthealth/
https://twitter.com/signanthealth
View original content to download multimedia:http://www.prnewswire.com/news-releases/signant-health-partners-with-propeller-health-to-gather-accurate-data-on-asthma-and-copd-medication-use-300982631.html
SOURCE Signant Health
