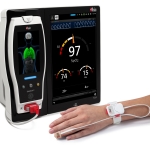
Masimo Announces FDA Clearance of Continuous RRp® Monitoring

IRVINE, Calif.–(BUSINESS WIRE)–Masimo (NASDAQ: MASI) announced today that continuous RRp® (respiration rate from the photoplethysmograph) monitoring of adult and pediatric patients with Rad-97®, Radical-7®, and Radius-7® Pulse CO-Oximeters® has received FDA clearance. With this clearance, both continuous and spot-check RRp are now available in the US, supported in a variety of pulse oximetry sensors and configurations, including the new non-cabled, tetherless, wearable Radius PPG™.
The availability of continuous RRp adds to Masimo’s diverse portfolio of respiration rate monitoring modalities – acoustic respiration rate (RRa®), NomoLine® capnography (RRc™), and now photoplethysmographic respiration rate (RRp®) – to help clinicians ensure they have the right tools for each patient scenario.
Determining RR, or the number of breaths taken per minute, in many situations typically requires manually counting breaths with a timer and then converting to a rate per minute, or being fitted with chest leads or straps that can be inconvenient. Acoustic respiration rate, RRa, has been shown to be an accurate1,2, reliable1, easy-to-use1, and easy-to-tolerate1,3 method of monitoring respiration rate on a continuous basis. If RRa or RRc is not available, however, for patients whose arterial oxygen saturation (SpO2) is already being monitored using Masimo SET®, RRp offers a convenient way to accurately obtain RR. RRp is particularly well suited to lower acuity settings like the general ward, where patients are less likely to have respiration rate monitoring technologies available. In scenarios where the ability to detect respiratory pause is important, such as during surgery or recovery after surgery, RRp should not be used; RRa or RRc is more appropriate.
With breathing difficulty generally considered one of the earliest signs of patient deterioration, Masimo hopes that the availability of RRp may be able to play a role in assisting clinicians and public health officials as they seek to combat respiratory-related illnesses, including the coronavirus COVID-19, especially when applying an additional sensor is not an option. In addition to RRp, RRc, and RRa, Masimo offers a wide range of noninvasive and continuous monitoring technologies – including arterial oxygen saturation (SpO2), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and total hemoglobin (SpHb®), as well as spot-check thermometry with the non-contact infrared thermometer TIR-1™ – designed to help clinicians monitor key physiological characteristics throughout the continuum of care, whether at the doctor’s office, during triage in the emergency room, on the general floor, or in the ICU. To support these technologies, Masimo provides a variety of single-patient-use sensors (and non-contact technology in the case of TIR-1), which help mitigate the risk of patient-to-patient transmission of communicable diseases.
Alongside RRp, Masimo SET® sensors offer Measure-through Motion and Low Perfusion™ SET® pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 SET® is estimated to be used on more than 150 million patients a year5 and is the primary pulse oximetry at 9 of the 10 hospitals that top the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.6
Joe Kiani, Founder and CEO of Masimo, said, “We aim to provide clinicians with the best monitoring tools so that they can provide the best care possible – which means recognizing that a monitoring method that works particularly well in one patient scenario may not be available or be the best choice in another. With the introduction of continuous RRp to our devices in the US, we are finally able to give clinicians in our home country a powerful third way to monitor respiration rate continuously, complementing other methods with a convenient and cost-effective single-sensor solution.”
@MasimoInnovates | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,7 improve CCHD screening in newborns,8 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.9-11 Masimo SET® is estimated to be used on more than 150 million patients in leading hospitals and other healthcare settings around the world,5 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.6 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Macknet MR et al. Accuracy and Tolerance of a Novel Bioacoustic Respiratory Sensor in Pediatric Patients. Anesthesiology. 2007;107:A84 (abstract).
- Goudra BG et al. Comparison of Acoustic Respiration Rate, Impedance Pneumography and Capnometry Monitors for Respiration Rate Accuracy and Apnea Detection during GI Endoscopy Anesthesia. Open J Anesthesiol. 2013;3:74-79.
- Patino M et al. Accuracy of Acoustic Respiration Rate Monitoring in Pediatric Patients. Paediatr Anaesth. 2013 Sep 3.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo RRp®, RRa®, NomoLine®, RRc™, and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo RRp, RRa, NomoLine, RRc, and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contacts
Media Contact:
Masimo
Evan Lamb
949-396-3376
elamb@masimo.com