South Korean IVD Company, SUGENTECH’s, COVID-19 IgM-IgG Rapid Test Listed on FDA
SUGENTECH’s COVID-19 IgM&IgG 5-10 minutes rapid test kit is listed on the U.S. FDA’s database and can be used in the U.S., as stated in Section IV.D of the FDA’s Policy for Diagnostic Tests for Coronavirus Disease-2019, and is planning to go further to seek EUA from U.S. FDA
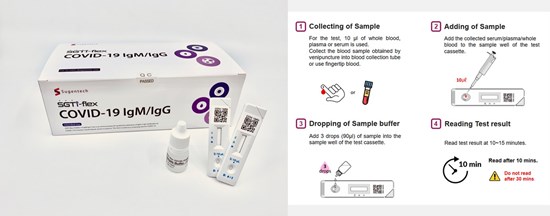
Seoul, South Korea–(Newsfile Corp. – April 8, 2020) – ‘SGTi-flex COVID-19 IgM&IgG’ is an immunochromatographic test kit for the qualitative determination of COVID-19’s IgM and IgG antibodies in whole blood (finger prick or venous), serum or plasma. The kits are accurate and easy to use, and results can be observed with the naked eye within 5-10 minutes. It has already received CE-IVD certification and is approved for exports from South Korean MFDS, and it showed 94.4% accuracy in clinical trials, which has been done with 250 samples in Daegu, South Korea.
SGTi-flex COVID-19 IgM&IgG test kit
To view an enhanced version of this graphic, please visit:
https://orders.newsfilecorp.com/files/7055/54277_ddbdced1943c090f_001full.jpg
‘SGTi-flex COVID-19 IgM&IgG’ is already being distributed in many countries in the EU and Asia, and in recent field tests in many renowned labs, including LMZ Dr. Risch Group in Switzerland, ‘SGTi-flex COVID-19 IgM&IgG’ has shown prominent performance in the neutralization test and been recognized even significant detection of early patient with IgM. SUGENTECH is planning to go further to seek EUA from the U.S. FDA.
Limits of RT-PCT for COVID-19
The real-time PCT (RT-PCR) test seeking the COVID-19 specific virus suffers from some inherent drawbacks such as 1) limitation of good sample patients (recent study tells that the upper respiratory sample has 30% sensitivity), 2) the need for specially trained technicians and equipped certified labs to run the test, and 3) high level of need in resources, e.g. cost and time.
Moreover, it has been reported that many COVID-19 virus-shedding patients are asymptomatic or have very few symptoms, and the period of virus shedding starts very early and stays so long, even when the virus titer is quite low.
‘SGTi-flex COVID-19 IgM&IgG’ is a serological (blood) rapid test.
‘SGTi-flex COVID-19 IgM&IgG’ detects the presence of COVID-19 specific lgM & lgG antibodies, which are specific proteins made within days after infection, even in patients that don’t have symptoms. The antibodies detected by this test indicate that a person had an immune response to COVID-19, whether symptoms developed from infection or the infection was asymptomatic. Antibody test results are important in detecting infections with few or no symptoms. It covers the entire course of infection and improves the overall diagnosis of COVID-19 in acute phases of illness.
It is very important to diagnose COVID-19 to defend against the expansion of COVID-19. ‘SGTi-flex COVID-19 IgM/IgG’ helps communities to identify infection and isolate people without symptoms but suspected to be infected by COVID-19.
About SUGENTECH
SUGENTECH is a South Korean IVD company and is listed on the Korean Stock Exchange (KOSDAQ: 253840). For more information, please visit: http://sugentech.com or contact info@sugentech.com.
MEDIA CONTACT:
Jongyoon Park, CFO/Managing Director
jypark@sugentech.com
Related Files
SGTi-flex COVID-19 IgM & IgG test_Clinical Evaluation.pdf
To view the source version of this press release, please visit https://www.newsfilecorp.com/release/54277
