AliveCor to Provide QTc Measurement for Clinicians Treating COVID-19 Patients
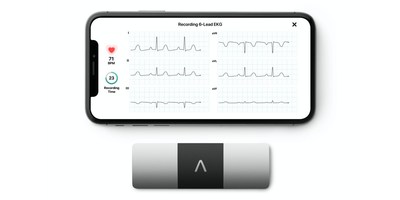
MOUNTAIN VIEW, Calif., April 13, 2020 /PRNewswire/ — AliveCor, the leader in AI-based, personal ECG technology, today announced it will offer a QTc measurement service on ECGs from AliveCor’s KardiaMobile 6L, the world’s only FDA-cleared six-lead personal ECG. This service will be offered for the duration of the COVID-19 emergency.
Starting today, medical professionals can use KardiaMobile 6L for patients, both in-hospital and at home, to evaluate QTc. ECG data will flow directly from AliveCor’s clinician platform to a nationally-known independent diagnostic testing facility, where QTc values will be determined and rapidly returned to front-line care providers.
Unlike most personal ECGs, KardiaMobile 6L records Lead II data, which provides a far more accurate view of the QTc interval than the Lead I view. Prolonged QTc can, in some cases, lead to a fatal side effect called drug-induced sudden cardiac death (DI-SCD). This potential side effect is associated with the use of several medications now being used in the treatment of COVID-19. As a result, close monitoring of QTc is required once a patient is started on these medications.
“This new service allows a patient’s healthcare team to access QTc measurements in rapid order,” said AliveCor CEO, Priya Abani. “Our aim is to help reduce the burden on our already strained healthcare system, so that clinicians can focus on treating patients. By delivering this service, we are doing just that.”
QTc measurement can be enabled for AliveCor professional healthcare customers beginning today. To learn more, please visit: alivecor.com/clinicians.
About AliveCor
AliveCor, Inc. is transforming cardiological care using deep learning. The FDA-cleared KardiaMobile 6L device is the most clinically validated personal ECG solution in the world. KardiaMobile provides instant detection of Atrial Fibrillation, Bradycardia, Tachycardia, and Normal rhythm in an ECG. Kardia is the first AI enabled platform to aid patients and clinicians in the early detection of atrial fibrillation, the most common arrhythmia and one associated with a highly elevated risk of stroke. AliveCor’s enterprise platform allows third party providers to manage their patients and customers’ heart conditions simply and profitably using state-of-the-art tools that provide easy front-end and back-end integration to AliveCor technologies. AliveCor was named the No.1 artificial intelligence company in Fast Company’s Top 50 Most Innovative Companies. AliveCor is a privately-held company headquartered in Mountain View, Calif. “Consumer” or “Personal” ECGs are ECG devices available for direct sale to consumers. For more information, visit alivecor.com.
Contact: press@alivecor.com
View original content to download multimedia:http://www.prnewswire.com/news-releases/alivecor-to-provide-qtc-measurement-for-clinicians-treating-covid-19-patients-301039163.html
SOURCE AliveCor

