Masimo Announces FDA Clearance of Centroid™

Wearable, Wireless Patient Orientation, Activity, and Respiration Sensor Helps Clinicians Monitor Patient Position and Respiration Rate
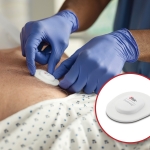
IRVINE, Calif.–(BUSINESS WIRE)–Masimo (NASDAQ: MASI) announced today that Centroid™, a wearable, wireless patient orientation, activity, and respiration rate sensor, has received FDA clearance. Centroid helps clinicians monitor patient position to avoid preventable pressure ulcers, and can alert clinicians to sudden movements such as fall-like events. In addition, Centroid detects chest movements to continuously provide respiration rate, assisting clinicians with additional data that may inform care decisions.
Centroid pairs with the Root® Patient Monitoring and Connectivity Platform using Bluetooth® to track a patient’s posture, orientation, and activity, providing the ability to monitor patient position and detect changes in position. The data transmitted by Centroid can be displayed in various formats on Root, giving clinicians multiple ways to assess adherence to protocols regarding tissue stress and to tailor care to the specific needs of each patient.
Pressure sores affect nearly 2.5 million patients per year in U.S. hospitals alone, and approximately 60,000 of those patients die as a direct result.1 Centroid is indicated for the orientation monitoring of patients who may be susceptible to pressure ulcers, by tracking patient movement and activity using an accelerometer and gyroscope. Centroid can identify a patient’s position and orientation to the nearest degree, with alerts based on the duration in a static position to help clinicians adhere to hospital patient turn protocols. Centroid also features customizable alarm zones to help avoid patient positions that could negatively impact recovery time. Unlike simple time-based rotation protocols, Centroid uses the cumulative time spent in each position, as well as existing sore data, to calculate relative risk, displayed on the Root screen using color-coded markers, helping clinicians identify the potential severity of tissue stress for each position—and ultimately, helping to guide clinical decisions about the most appropriate, least risky positions for each patient.
In addition, because Centroid can identify whether a patient is lying down, standing, sitting upright, walking, or may have fallen, it can notify clinicians of a sudden change in position that might provide early warning of a potential fall, by alerting them when clinician-defined movement thresholds are crossed. Its respiration rate performance, validated against manually scored capnogram respiratory measurements, is accurate to within 3 respirations per minute (rpm) in the range of 8 to 35 rpm.
The Centroid single-patient-use sensor is ergonomically designed for application on the chest using flexible, lightweight material for patient comfort, with a gentle adhesive that supports continuous use during daily activities. Each battery-operated sensor is designed to last four days, minimizing the need for frequent replacement.
Joe Kiani, Founder and CEO of Masimo, said, “We are committed to using our expertise in signal processing and sensor design to develop new ways to provide the highest quality, most relevant data to clinicians in the most intuitive, useful formats, and Centroid, coupled with Root’s rich high-resolution display, is a great example of this. We hope that by helping to automate the process of tracking and making decisions about patient position, we can help clinicians reduce the frequency and severity of pressure ulcers, and ultimately improve patient outcomes.”
@MasimoInnovates | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Preventing Pressure Ulcers in Hospitals. Agency for Healthcare Research and Quality. https://www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu1.html. Accessed 23 Apr 2020.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO 2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Centroid™ and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo Centroid and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contacts
Media Contact:
Masimo
Evan Lamb
949-396-3376
elamb@masimo.com