Immunicom Announces Promising Preliminary Data from its Immunopheresis® Study in Late-Stage Cancer and Metastatic Melanoma Patients Presented at 2021 SITC Congress

Immunicom’s highly selective extracorporeal blood-filtering column shown to be safe and well-tolerated in treating late-stage cancer patients who fail multiple lines of standard chemo and immunotherapies
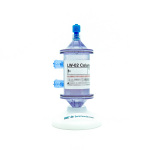
SAN DIEGO–(BUSINESS WIRE)–#biotech—Immunicom, Inc., a clinical-stage biotech pioneering subtractive therapies for advanced cancers, presented promising preliminary data from a clinical trial investigating Immunopheresis® therapy for resistant melanoma and other solid cancers at the Society for Immunotherapy of Cancer (SITC) 2021’s 36th annual meeting. The clinical trial is being conducted at Sheba Medical Center and uses Immunicom’s proprietary, subtractive LW-02 column to selectively remove immunosuppressive soluble tumor necrosis factor receptors (sTNF-Rs) via apheresis. By depleting TNF receptors from plasma, this therapy is designed to free the cytokine TNF-alpha, thereby unleashing its potent anti-cancer effects.
The basket oncology clinical trial (NCT04142931; n =30 patients), which is being conducted under the direction of Dr. Ronnie Shapira, MD, and Prof. Gal Markel, MD PhD, is evaluating the safety and the clinical effectiveness of Immunopheresis as a monotherapy, and in combination with Bristol Myers Squibb’s anti-PD-1 immune checkpoint inhibitor, OPDIVO® (nivolumab), in heavily pretreated patients. Preliminary data were presented from Part A (Immunopheresis therapy alone) from six patients—three with melanoma, and three with triple negative breast cancer (TNBC)—who have failed multiple prior lines of standard chemo and immunotherapies. Of these six heavily pretreated patients, three completed the 12-week study regimen, and three were withdrawn due to clinical progression. Two patients are still alive after a year (median overall survival 26.6 weeks). Immunopheresis therapy was well-tolerated in these late-stage patients, and no treatment-related serious adverse events were reported. The therapy was demonstrated to significantly reduce the levels of sTNF-Rs in plasma. Immune profiling of blood and tumor specimens showed evidence of upregulation of protein biomarkers and cell populations that are associated with improved activity of the immune system. Specifically, the tumor specimens showed two developments: 1) an increased infiltration of CD8+ T cells, which are known to have anti-tumor cytotoxic effects, and 2) the expression of immune checkpoint proteins PD1 and PD-L1 that show evidence of turning “cold” tumors into “hot” tumors. Part B of the study—evaluating Immunopheresis therapy in combination with nivolumab—is currently ongoing. The SITC 2021 Congress abstract is available on Immunicom’s Publications page.
“Our preliminary data, which show renewed immune modulation and intra-tumoral antitumor activity, support our hypothesis that Immunopheresis therapy has the potential to overcome immunotherapy resistance, which could be a significant breakthrough in refractory cancer patient care,” said Professor Gal Markel, former Director of the Ella Lemelbaum Institute for Immuno-Oncology, and now Director of Davidoff Center & Deputy Director General, Rabin Medical Center at Clalit Health Services. “Immunicom’s innovative subtractive treatment approach to neutralize cancer’s ability to block the patient’s natural immune-defense mechanisms is especially attractive in these heavily pretreated patients, and it offers the potential for achieving much better clinical outcomes with fewer treatment side effects,” added Dr. Ronnie Shapira, a melanoma expert, and Head of the Onco-Gynecological Cancer Unit at the Ella Lemelbaum Institute for Immuno-Oncology.
Speaking to the announcement, Amir Jafri, Immunicom’s Founder and CEO, added, “The clinical trial at Sheba Medical Center is one of three ongoing, groundbreaking clinical trials to assess Immunicom’s subtractive therapies. Right now, our other clinical trials are evaluating the LW-02 column for treating additional multiple cancers, including metastatic TNBC, non-small cell lung cancer, melanoma, and renal cell carcinoma.”
Immunicom’s various clinical trials are being conducted in collaboration with world-renowned research organizations and thought leaders, including:
Poland – at Jagiellonian University of Krakow Hospital, under the direction of Principal Investigator, Professor Piotr Wysocki, MD, PhD
Turkey – at Acıbadem Altunizade Hospital (Istanbul), a member of the Acıbadem/IHH Healthcare Group, under the direction of Principal Investigator, Prof. Dr. Gokhan Demir, MD, PhD
Subtractive Therapy – Immunopheresis® and the LW-02 Column
Immunicom’s innovative Immunopheresis approach uses the LW-02 column to extract specific immune-suppressive cytokine receptors produced by cancer tumors. Selective removal of these targeted cytokine receptors is intended to neutralize cancer’s ability to block a patient’s natural immune defense mechanisms—which are significantly compromised in late-stage, metastatic disease—thereby re-energizing the immune system to aggressively fight the cancer. Immunopheresis is a “subtractive therapy” in contrast to drugs that are “additive.” Subtractive therapy is designed to avoid the side effects, toxicity, and negative impact on a patient’s quality of life that are typical of other cancer treatments.
Based on Immunicom’s clinical program, the LW-02 column could be used either in combination with other therapies, or as a stand-alone treatment. The LW-02 Immunopheresis column has already received Breakthrough Device Designation for stage IV metastatic cancers from the U.S. Food and Drug Administration (FDA) and European regulatory clearance (CE Mark certification) for use in adults with advanced, refractory TNBC. Immunicom has obtained ISO 13485 certification for its manufacturing and related quality systems.
For an overview of Immunopheresis and how this breakthrough technology works, watch Immunicom’s How it Works video.
Immunopheresis and the LW-02 column is considered an investigational therapy by the U.S. FDA and other regulatory authorities. The clinical efficacy of the LW-02 column has not yet been demonstrated. Clinical investigations evaluating the clinical efficacy of the LW-02 column for TNBC are ongoing.
About Immunicom
Immunicom, Inc. creates novel immunotherapies designed to treat a variety of diseases using its breakthrough Immunopheresis® technology platform to improve patient access and affordability. The privately held medical technology company develops innovative, non-pharmaceutical approaches for treating cancer, autoimmune disorders, and inflammatory and renal diseases. Immunicom’s revolutionary blood-filtering Immunopheresis technology has the potential to effectively treat a wide variety of cancer types, including those that have not responded to other treatment strategies, with possibly fewer side effects. Immunicom’s lead product, the LW-02 column, has received U.S. FDA Breakthrough Device designation for stage IV metastatic cancer and European regulatory clearance (CE Mark certification) for use in adults with advanced, refractory, triple negative breast cancer (TNBC). Immunopheresis is currently being evaluated in several global oncology trials for multiple cancers. Immunicom is headquartered in San Diego, CA with operations in Philadelphia, PA, Houston, TX, and Krakow, Poland.
About Sheba Medical Center at Tel Hashomer
Sheba Medical Center is dedicated to providing exceptional healthcare to all patients from all over the world. It is the largest hospital in Israel and sets the standard of excellence in patient-focused care. Sheba Medical Center’s state-of-the-art facilities are located on a comprehensive, all-inclusive campus with a full range of medical divisions and specialties. Sheba has been included in the prestigious Newsweek annual investigative report as one of the top 10 hospitals worldwide. The endorsement honors Sheba as a leading force in medical science and biotechnological innovation—skillfully adapting to the challenges of technology while always providing the highest quality of patient care.
Forward-Looking Statements
This press release contains certain forward-looking statements regarding Immunicom device capabilities. All such statements are based upon current Immunicom expectations and involve a number of business and technical risks and uncertainties that could cause actual results to differ materially from anticipated results described, implied or projected in any forward-looking statement, including, without limitation, clinical trial results, regulatory approvals, unexpected changes in technologies, uncertainties inherent in product development and commercialization, intellectual property protection, and the ability of our products to gain market acceptance.
Contacts
Stephen Prince
publicrelations@immunicom.com
657-202-6040