Halberd Moves Closer to Proving Its Patented Technologies Eliminate Contributors to Neurodegenerative Diseases
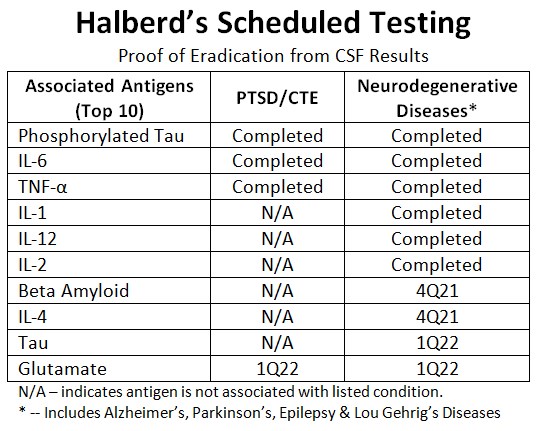
Latest Testing Eliminates Interleukin-2 From Cerebral Spinal Fluid
Jackson Center, Pennsylvania–(Newsfile Corp. – December 20, 2021) – Halberd Corporation (OTC Pink: HALB) has been able to successfully eliminate Interleukin-2 (IL-2) from synthetic cerebral spinal fluid (CSF) in preliminary testing. Halberd also successfully replicated its previous successful elimination of each of the inflammatory cytokines listed as complete in the table below. Interleukin-2 is a pro-inflammatory cytokine whose role in Alzheimer’s Disease is not very well established, particularly given the multiplicity of bio factors implicated in this devastating disease. It may cause neurodegeneration as well as provide neuroprotection, depending on the level. Maintaining the appropriate level of IL-2, and other inflammatory cytokines in CSF is critical to neuro-health.
Dr. Mitchell S. Felder, Halberd’s Chief Technology Officer and a board-certified attending neurologist stated, “The ability to precisely control the exact level of IL-2 in cerebrospinal fluid may be another important step in halting the progression of Alzheimer’s Disease; especially when this ability is combined with the precise control of the concentrations of phosphorylated- Tau, beta-Amyloid and other contributing inflammatory cytokines.”
To view an enhanced version of this graphic, please visit:
https://healthtechnologynet.com/wp-content/uploads/2021/12/108080_7141c858448f48ac_001full.jpg
William A. Hartman, Halberd Corporation’s Chairman, President & CEO, added, “The significance of these test results is that Halberd’s technology can be used to precisely control the levels of inflammatory cytokines and neurotransmitters to inhibit progression, and aid in the treatment, of various neurodegenerative diseases, such as Alzheimer’s, Lou Gehrig’s Disease, PTSD, CTE and epilepsy, among others. No other approach gives this kind of precise control which is imperative in maintaining a healthy balance of needed elements in the body.”
Hartman continued, “This tool has the potential to revolutionize medicine and the treatment of various neurologic and non-neurologic diseases and conditions. The potential of this as an effective treatment for Alzheimer’s, PTSD and CTE is a huge step forward for medicine. Additionally, since Alzheimer’s Disease shares a number of common inflammatory cytokines with several other diseases, such as cancer, this could lead to an effective treatment for those other diseases which also have eluded removal of the basic causes of the disease.”
To get the latest news on Halberd’s exciting developments, including our ongoing disease eradication accomplishments, subscribe by submitting this form.
(https://halberdcorporation.com/contact-us/)
For more information please contact:
William A. Hartman
w.hartman@halberdcorporation.com
support@halberdcorporation.com
www.halberdcorporation.com
Twitter: @HalberdC
About Halberd Corporation.
Halberd Corporation (OTC Pink: HALB), is a publicly traded company on the OTC Market, and is in full compliance with OTC Market reporting requirements. Since its restructuring in April of 2020, Halberd has obtained exclusive worldwide rights to three issued patents and has filed nineteen related provisional, PCT, or utility patent applications to enhance its value to its stockholders and to attract the interests of potential development partners.
Safe Harbor Notice
Certain statements contained herein are “forward-looking statements” (as defined in the Private Securities Litigation Reform Act of 1995). The Company cautions that statements, and assumptions made in this news release constitute forward-looking statements and makes no guarantee of future performance. Forward-looking statements are based on estimates and opinions of management at the time statements are made. These statements may address issues that involve significant risks, uncertainties, estimates made by management. Actual results could differ materially from current projections or implied results. The Company undertakes no obligation to revise these statements following the date of this news release.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/108080
