Red Light Holland Psilocybin Truffles, Sold in The Netherlands, Complete Second Evaluation Under a Health Canada Approved cGMP Laboratory
Red Light Holland and CCrest Laboratories continue to demonstrate their strong commitment to the highest regulatory Health Canada compliance standards, as the two synergistic companies applaud, and are paying close attention to, Health Canada’s updated Special Access Program (SAP)
Toronto, Ontario–(Newsfile Corp. – January 6, 2022) – Red Light Holland Corp. (CSE: TRIP) (FSE: 4YX) (OTC Pink: TRUFF) (“Red Light Holland” or the “Company“), an Ontario-based corporation engaged in the production, growth and sale of a premium brand of magic truffles, is pleased to announce that it has received the second report, produced by Shaman Pharma Corp. and CCrest Laboratories Inc. under a Health Canada Controlled Drugs & Substances license. This report was focused on perfecting potency assays and identifying variables such as water content and size that might influence the amount and the characteristics determining active ingredients (psilocybin and psilocin) in the truffles as a step towards creating a standardized consistent dose from naturally occurring psychoactive truffles.
“We continue to move towards creating a standardized consistent dose from naturally occurring psilocybin truffles that can potentially benefit both the hopeful recreational and medicinal markets,” said Todd Shapiro, CEO and Director of Red Light Holland. “We are learning through market research and anecdotal movements that many people prefer naturally occurring psilocybin over synthetics – so it’s exciting that Red Light Holland with the help of Shaman Pharma are once again working aggressively within all legal means, and adding to the scientific knowledge of these natural products. We hope the Canadian Government will see the need to provide patients, who are approved to use natural psilocybin, with an ability to access our tested products of known dosage.”
“The progress we have made in the year 2021 was simply groundbreaking which positioned our companies to start this new year with incredible momentum. We look forward to continuing and expanding our collaboration with Red Light Holland.” said Alex Grenier, CEO of Shaman Pharma and President of CCrest Laboratories. “The timely news regarding the Special Access Program is in line with our expectations and we are fully committed to exceeding the exemplary regulatory standards put forward by Health Canada, hoping it will inspire the health authorities of other countries to follow without hesitation.”
Now that a precise and specific analysis method (SP1-173-L) has been determined next steps will be taken to test its robustness and accuracy. Other substances were also identified in the truffles and more bibliography will be needed to identify other alkaloid compounds that might be present in the truffles such as Norbaeocystin, Baeocystin and Aeruginascin which will help research the possibility of an “entourage effect” in naturally occurring truffles.
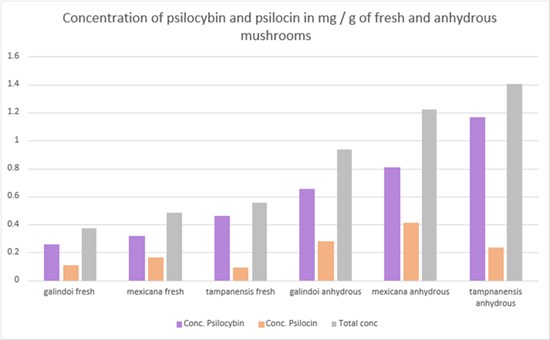
From the report, it can be inferred that the size is not affecting the concentrations of psilocybin and psilocin, but the water content is an essential factor. In the case of large-sized truffles (Galindoi and Tampanensis) removal of water content, without degrading psilocybin, is a challenging task which in turn affects the extract concentrations. It can be observed from these results that the laboratory was successfully able to bring the raw material to their anhydrous state while maintaining the same ratio of active ingredients.
Figure 1
To view an enhanced version of Figure 1, please visit:
https://orders.newsfilecorp.com/files/2017/109252_a4a3dcc8d47728da_001full.jpg.
Future analysis will also expand research using scientific methods applied in pharmaceutical drug development, for instance determining the exact influence of light on the degradation of psilocybin and psilocin by doing a forced degradation study, and performing stability studies under various conditions. These studies can illustrate the chemical stability of the molecule which further facilitates the development of stable experimental design and suitable storage conditions. This information will aid in the development of lab-scale production projects to provide practitioners with quality-controlled psilocybin for their patients.
On January 5, 2022, the Controlled Substances Directorate of Health Canada announced that amendments were published and came into force immediately to restore the possibility for practitioners who are allowed to prescribe drugs to request access to restricted drugs, including psilocybin, through Health Canada’s Special Access Program (SAP). With the regulations now amended, practitioners who are allowed to prescribe drugs can, on behalf of patients with serious or life-threatening conditions, request access to restricted drugs through the SAP when other therapies have failed, are unsuitable, or are not available in Canada, among other conditions.
While all SAP requests will continue to be assessed on a case-by-case basis, and there is no guarantee that access to restricted drugs will be granted through the SAP, this much anticipated step forward validates the importance of the work performed at CCrest Laboratories with psilocybin truffles from Red Light Holland. Canada is leading the way in providing practitioners with a path for patients to access psilocybin, and it is critical to make it readily available through a legal and quality-controlled supply chain, and that practitioners can rely on specific, precise and consistent dosage.
Red Light Holland continues to establish itself as a leader in the recreational sector and push for legal, responsible and safe access to natural psychedelic truffles/mushrooms while Scarlette Lillie Science and Innovation pursues research and development, technology, and applied science.
About Red Light Holland
Red Light Holland is an Ontario-based corporation engaged in the production, growth and sale (through existing Smart Shops operators and an advanced e-commerce platform) of a premium brand of magic truffles.
For additional information on the Company:
Todd Shapiro
Chief Executive Officer & Director
Tel: 647-643-TRIP (8747)
Email: todd@redlight.co
Website: www.RedLight.co
About Shaman Pharma Corp.
Shaman Pharma is a federally registered Canadian corporation with the mission to power outstanding psychedelic life science innovation. Accelerating time-to-market through its portfolio of assets, Shaman launches and consolidates revenue-driven pharma-biotech life sciences ventures focused on supplying psychedelic drugs & novel active ingredients.
Forward-Looking Statements
Neither the Canadian Securities Exchange nor its Market Regulator (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this release.
Certain information set forth in this news release may contain forward-looking statements that involve substantial known and unknown risks and uncertainties, certain of which are beyond the control of Red Light Holland. Forward-looking statements are frequently characterized by words such as “plan”, “continue”, “expect”, “project”, “intend”, “believe”, “anticipate”, “estimate”, “may”, “will”, “potential”, “proposed” and other similar words, or statements that certain events or conditions “may” or “will” occur. These statements are only predictions. Readers are cautioned that the assumptions used in the preparation of such information, although considered reasonable at the time of preparation, may prove to be imprecise and, as such, undue reliance should not be placed on forward-looking statements. Forward-looking statements include, but are not limited to: statements with respect to the Company creating a standardized consistent dose from naturally occurring psychoactive truffles; statements with respect to Health Canada’s Special Access Program, including the Company’s expectations with respect to exceeding any potential regulatory standards set by such program; statements with respect to further evaluation and testing of the Company’s naturally occurring psilocybe truffles by CCrest Laboratories for scientific and medical purposes; the potential of the Company’s products being used for scientific and medical purposes; the Company’s ability to establish itself as the leader in the recreational psychedelics sector.
Forward-looking information is based on a number of key expectations and assumptions made by Red Light Holland, including without limitation: the COVID-19 pandemic impact on the Canadian economy and Red Light Holland’s business, and the extent and duration of such impact; no change to laws or regulations that negatively affect Red Light Holland’s business; there will be a demand for Red Light Holland’s products in the future; no unanticipated expenses or costs arise; the Company will be able to continue to develop products that are allowed to be imported and sold under Health Canada’s import permit; and the partnership with Shaman Pharma Corp. will help Red Light Holland to achieve its business goals. Although the forward-looking information contained in this news release is based upon what the Company believes to be reasonable assumptions, it cannot assure investors that actual results will be consistent with such information.
These statements involve known and unknown risks, uncertainties and other factors, which may cause actual results, performance or achievements to differ materially from those expressed or implied by such statements, including but not limited to: the inability of the Company to continue as a going concern; the inability of the Company to obtain all necessary governmental and/or other regulatory approvals, licenses, and permits necessary to operate and expand the Company’s facilities; the effect of regulatory and/or political change and its effect on the legislation and regulations surrounding the psychedelics industry including SAP; negative perception of the medical-use and adult-use psilocybin industry; the inability of CCrest to complete the planned testing of the Company’s products; the inability of the Company to create a standardized dose; the potential unviability of psilocybin for medical and/or scientific purposes; the inability of the Company to continue its growth; the Company’s limited operating history; reliance on management; the Company’s requirements for additional financing; and competition for mental health and wellness investments.
Readers are cautioned that the foregoing list is not exhaustive. Readers are further cautioned not to place undue reliance on forward-looking statements, as there can be no assurance that the plans, intentions or expectations upon which they are placed will occur. Such information, although considered reasonable by management at the time of preparation, may prove to be incorrect and actual results may differ materially from those anticipated.
Forward-looking statements contained in this news release are expressly qualified by this cautionary statement and reflect the Company’s expectations as of the date hereof and are subject to change thereafter. The Company undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, estimates or opinions, future events or results or otherwise or to explain any material difference between subsequent actual events and such forward-looking information, except as required by applicable law.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/109252
