Signifier Medical Technologies is Granted HCPCS Codes for eXciteOSA to Help Patients Access Innovative Technology

CMS establishes new Level II HCPCS codes to describe eXciteOSA, the first-ever daytime treatment for obstructive sleep apnea
BOSTON–(BUSINESS WIRE)–Signifier Medical Technologies LLC, a Boston-based medical technology company, announced today that the Centers for Medicare & Medicaid Services (CMS) established two new Level II Healthcare Common Procedure Coding System (HCPCS) codes to describe eXciteOSA, the first-ever FDA authorized de-novo device for daytime treatment of mild obstructive sleep apnea (OSA) and primary snoring. The CMS coding decision was published on February 16, 2022 and the following codes will become effective on April 1, 2022:
|
HCPCS Code |
Description |
|
K1028 |
Power source and control electronics unit for oral device/appliance for neuromuscular electrical stimulation of the tongue muscle for the reduction of snoring and obstructive sleep apnea, controlled by phone application. |
|
K1029 |
Oral device/appliance for neuromuscular electrical stimulation of the tongue muscle, used in conjunction with the power source and control electronics unit, controlled by phone application, 90-day supply. |
“We welcome CMS’ decision to establish two new codes to describe the eXciteOSA device”, said Akhil Tripathi, Chief Executive Officer at Signifier. “With over 3,000 patients treated with eXciteOSA, we have seen strong demand for our therapy. Establishing codes marks an important milestone for physicians, health systems, DME suppliers, payers and patients alike, and will enable Signifier to demonstrate further the real world benefits of improved outcomes, cost savings and significantly improved quality of care for patients. Reimbursement will help patients in the US who suffer from sleep disordered breathing with an option to obtain a new, effective daytime treatment.”
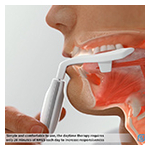
OSA is a progressive disease, often starting with primary snoring, affecting nearly 1 billion adults aged 30 to 69 globally.1 Used for only 20 minutes per day during the initiation period, and then twice per week thereafter for maintenance, eXciteOSA targets a primary root cause of mild OSA by delivering neuromuscular electric stimulation to increase tongue muscle endurance.
“HCPCS codes play a critical role in providing patients with access to new medical devices, often improving payment options and healthcare decisions,” said Dr. Marc Benton, a New Jersey-based pulmonologist and sleep medicine specialist. “The eXciteOSA is a non-invasive, easy to use therapy that does not require patients to wear any sort of device during the night. Once reimbursement is established, eXciteOSA will be easier to access for a broader and more diverse population of patients suffering from sleep apnea, and I’m excited to share the good news with all of them.”
The CMS decision can be viewed at: https://www.cms.gov/files/document/2021-hcpcs-application-summary-biannual-2-2021-non-drug-and-non-biological-items-and-services.pdf
About Signifier Medical Technologies
Signifier is a pioneer in addressing the root causes of sleep disordered breathing. We are focused on the development and commercialization of innovative and non-invasive solutions for patients with conditions such as Obstructive Sleep Apnea and primary snoring. Founded in 2015, Signifier is at the forefront of sleep therapy, with a mission to develop therapies that improve population health, increase the quality of patients’ experience and generate healthcare savings. Signifier has offices in London (UK), Needham (Massachusetts, USA) and Berlin (Germany).
About eXciteOSA®
eXciteOSA is a revolutionary daytime therapy device for sleep disordered breathing. Clinically proven to target a common root cause of OSA, eXciteOSA improves sleep quality, improves health and increases quality of life. Nearly one billion adults aged 30 to 69 years globally are estimated to suffer from OSA, which is a serious medical condition associated with health problems such as high blood pressure and increased risks of heart attack, stroke or death.1-10
A major underlying cause of OSA is that the upper airway muscles lack endurance during sleep and the tongue falls back, blocking the upper airway. By using neuromuscular electrical stimulation (NMES) to “exercise” the upper airway muscles, eXciteOSA works the intrinsic and extrinsic tongue muscles to improve endurance and prevent airway collapse during sleep.
Unlike other devices which are used while patients sleep, eXciteOSA is the first commercially available device used while awake. The full benefits of the daytime therapy are realized without patient use during sleep.
About Snoring and Obstructive Sleep Apnea
Nearly one billion adults aged 30 to 69 years are estimated to have OSA globally.1 OSA and snoring are problems for many people whose tongue muscles relax during sleep, causing airway collapse and decreased oxygen intake, which causes the sleeper to stop and restart breathing during sleep, often jolting them awake.15-16
OSA is a serious medical condition associated with health problems like high blood pressure and increased risks of heart attack, stroke, or death.2-9 OSA and snoring also affect patients’ partners and family members, consequently putting strain on relationships.15-16
For more information, please visit www.signifiermedical.com or www.exciteosa.com
References
- Benjafield, A. V., Ayas N T, Eastwood P R et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019; 7: 687-698.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342:1378–1384. [PubMed: 10805822]
- Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352– 360. [PubMed: 20625114]
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnoea as a risk factor for stroke and death. N Engl J Med. 2005; 353:2034–2041. [PubMed: 16282178]
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea- hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010; 182:269–277. [PubMed: 20339144]
- Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnoea: a 7-year follow-up. Am J Respir Crit Care Med. 2002; 166:159– 165. [PubMed: 12119227]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005; 365:1046–1053. [PubMed: 15781100]
- Peppard PE, Szklo-Coxe M, Hla KM, et al. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006; 166:1709–1715. [PubMed: 16983048]
- Kendzerska T, Gershon AS, Hawker G, et al. Obstructive sleep apnoea and incident diabetes: a historical cohort study. Am J Respir Crit Care Med. 2014; 190:218–225. [PubMed: 24897551]
- Johnson KG and Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J of Clinical Sleep Med 2010;6(2):131-7
- Baptista et al. Daytime Neuromuscular Electrical Therapy of Tongue Muscles in Improving Snoring. 2021
- Kotecha. et al. A novel intraoral neuromuscular stimulation device for treating sleep-disordered breathing. Sleep Breath. 2021.
- Data on File. Signifier Medical Technologies
- Davey, M. J. Epidemiological study of snoring from a random survey of 1075 participant. British Snoring and Sleep Apnoea Association. 2002; Available at: https://britishsnoring.co.uk/pdf/epidem.pdf
- Levy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 2015; 1: 15015.
- Luyster FS. Impact of Obstructive Sleep Apnea and Its Treatments on Partners: A Literature Review. J Clin Sleep Med. 2017;13(3):467-477.
Contacts
Frazer Hall at MEDiSTRAVA Consulting as the named contact
frazer.hall@medistrava.com
+44 (0) 203 928 6900)
marketing@signifiermedical.com
+1 (844) 645 3672
_1.jpg)

