iTolerance, Inc. Pre-Clinical Non-Human Primate Study Demonstrates Long-Term Success of Allogeneic Islet Implantation Without Chronic Immunosuppression for the Treatment of Diabetes
Data published in peer-reviewed journal, Science Advances
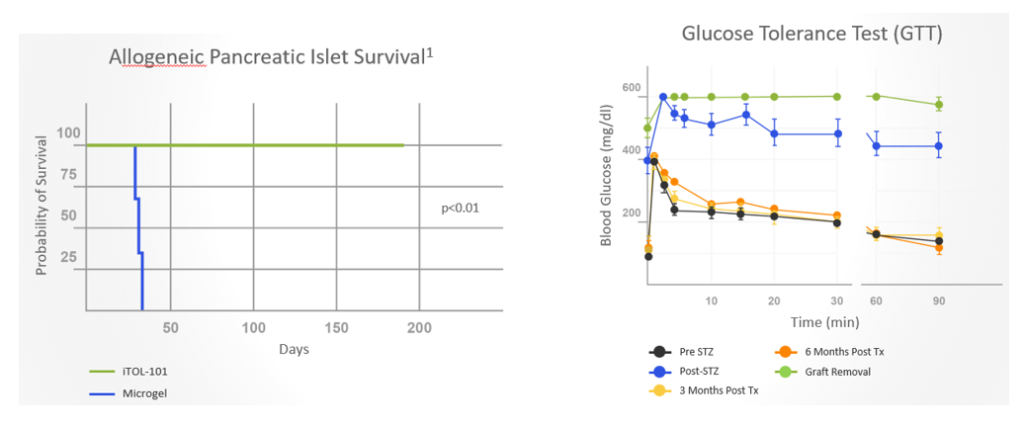
Co-implantation of allogeneic islets and streptavidin (SA)-FasL-presenting microgels sustained long-term (>6 months) survival of pancreatic islet cells and provided excellent glycemic control in non-human primates (NHPs) with diabetes without chronic immunosuppression
Following islet implantation, prompt glycemic control was achieved and maintained by all animals receiving SA-FasL-presenting microgels
Success in NHP model, the gold standard translational model for allogeneic transplantation, provides validation for advancement into human clinical studies
MIAMI, FL / ACCESSWIRE / May 16, 2022 / iTolerance, Inc. (“iTolerance” or the “Company”), an early-stage regenerative medicine company developing technology to enable tissue, organoid or cell therapy without the need for life-long immunosuppression, today announced the publication of positive pre-clinical results from a non-human primate (NHP) study evaluating co-implantation of allogeneic islets and SA-FasL-presenting microgels for the treatment of diabetes.
The manuscript titled, FasL-microgels induce immune acceptance of islet allografts in nonhuman primates[1], was published in the peer-reviewed Journal, Science Advances. The corresponding authors for the published manuscript are the Company’s Scientific Founders and Advisory Board members, Andrés J. García, PhD, F.B.S.E., Haval Shirwan, PhD, and James F. Markmann MD, PhD.
Anthony Japour, MD, Chief Executive Officer of iTolerance, commented, “The NHP data and its publication is a landmark milestone for iTolerance. Chronic immunosuppression carries significant long-term morbidity and mortality risks, including potential for cancer, decline in kidney function, and beta cell toxicity. Eliminating chronic immunosuppression could obviate the need for chronic prophylactic antiviral, antifungal, and antimicrobial agents prescribed to prevent serious infections with immunosuppression regimens. Therefore, demonstrating sustained long-term survival of pancreatic islet cells and glycemic control in non-human primates with diabetes without chronic immunosuppression is potentially game changing in the advancement of a possible cure for Type 1 Diabetes.”
Dr. Japour added, “We are grateful to our team of scientists for their work, the JDRF for providing valuable resources and support for the study, and Science Advances for publishing this important data. Now more than ever, we are dedicated to translating these findings to the clinic and are in ongoing discussions with the U.S. FDA to progress toward human clinical trials.”
Dr. García, the Company’s Scientific Co-Founder and Executive Director, Parker H. Petit Institute for Bioengineering and Bioscience Petit Director’s Chair in Bioengineering and Bioscience Regents Professor, George W. Woodruff School of Mechanical Engineering Georgia Institute of Technology, stated, “We are incredibly pleased with the results from this study. Achieving long-term survival of allogeneic grafts without chronic immunosuppression was, until now, an elusive goal. In this very stringent model of islet implantation, we were able to successfully demonstrate a reliable and safe tolerogenic regimen for diabetes without the use of chronic immunosuppression – an exciting finding. We believe this major advancement enables us to take a step toward developing a potential cure for Type 1 Diabetes.”
“The use of long-term immunosuppression medications remains the major hurdle for the use of cell and regenerative therapies,” added Camillo Ricordi, MD, Chief Scientist of iTolerance. “The data generated through this NHP study establishes that the approach we have developed may eliminate the need for chronic immunosuppression, a revolutionary approach that I believe will change the way that the scientific research community approaches regenerative medicine.”
Summary of Pre-Clinical Non-Human Primate Study for the Treatment of Diabetes
The NHP study investigated the immunomodulatory potential of the SA-FasL microgel technology in a pre-clinical streptozotocin (STZ)-induced diabetes NHP model in which allogeneic islets and SA-FasL microgels are co-implanted into the omentum, an area of fatty tissue attached to the stomach.
- Results demonstrated robust glycemic control, sustained C-peptide levels, and graft survival in non-human primates with diabetes for >6 months. Surgical extraction of the graft resulted in prompt hyperglycemia. In contrast, animals receiving microgels without SA-FasL under the same rapamycin regimen rejected islet grafts acutely.
- Graft survival was associated with increased number of regulatory FoxP3+ cells in the graft site with no significant changes in T cell systemic frequencies or responses to donor and third-party antigens, indicating localized tolerance.
- Immunostaining analyses of the graft site demonstrated the presence of FoxP3+ (a marker of Treg) cells at a higher frequency (cell count, intensity) in SA-FasL Microgel treated NHPs compared to Microgel subjects (FoxP3+ cell counts: p-value=0.0143; FoxP3+ intensity: p-value=0.043). This finding parallels studies conducted in mice with diabetes demonstrating a pivotal role for Treg in establishing SA-FasL-microgel-induced immune acceptance.
- NHPs treated with SA-FasL-presenting microgels did not reveal changes between pre- and post-transplant IFN-γ secreting cell numbers and anti-donor major histocompatibility complex (MHC) antibodies. In contrast, subjects that received control microgels generated antibodies against donor MHC molecules and exhibited increased CD8+ T cell proliferative responses to donor and third-party antigens.
- Subjects receiving allogeneic islets and SA-FasL microgels exhibited normal liver and kidney metabolic function and organ histology and continued to gain weight.
- This localized immunomodulatory strategy succeeded with unmodified islets and does not require long term immunosuppression, showing translational potential in β-cell replacement for treating diabetes.

Dr. Shirwan, from the Department of Child Health and Molecular Microbiology and Immunology and Director, Immunomodulation and Translational Research Program at the University of Missouri and a Scientific Co-Founder of the Company, commented, “The Fas receptor/Fas ligand (FasL) pathway plays a crucial role in activation-induced cell death and tolerance to self-antigens. In previously conducted mouse models, we demonstrated that co-implantation of SA-FasL-presenting microgels with unmodified allogeneic islets with a short course of rapamycin resulted in long-term engraftment and function in mice with diabetes. I am thrilled that we were able to translate that pre-clinical success to a NHP model, a model I believe is much more reflective of the potential outcome in humans. I look forward to helping advance this noteworthy breakthrough and unlocking its full potential for the treatment of Type 1 Diabetes and beyond.”
Dr. Ricordi added, “Most importantly, this biomaterial-based strategy offers off-the-shelf immunomodulatory capability without modification of donor islets, increasing its clinical relevance and breadth of potential clinical applicability. These findings are a major achievement that justifies the moving this technology forward towards clinical application in the most expedient and efficient way possible.”
Dr. Markmann, Scientific Co-Founder of iTolerance and Chief, Division of Transplantation Surgery and Director, Liver, Pancreas and Islet Transplant Programs and Director of Clinical Operations, MGH Transplant Center, said, “It is unusual to demonstrate such long-term success of allogeneic islet implantation in a non-human primate without the need for chronic immunosuppression. The simple, minimally invasive strategy used and encouraging results provide added confidence in the translatability of this technology to the clinic. Additionally, with the data seen to date, we believe this platform technology could be utilized in other areas of cell therapy and bring potential solutions to patients and physicians.”
The NHP study was made possible by funding and support from the JDRF. Study protocols were approved by the Institutional Animal Care and Use Committee at the Massachusetts General Hospital Research Institute.
About iTOL-100
The Company’s iTOL-100 platform technology is a biotechnology-derived Strepavidin-FasL fusion protein, a synthetic form of the naturally occurring protein FasL, mixed with a biotin-PEG microgel (SA-FasL microgel) that potentially allows convenient and effective co-administration with implanted cells or organoids to induce local immune tolerance without the need for life-long immunosuppression. In pre-clinical studies, iTOL-100 has been shown to establish durable, localized immune tolerance, allowing the implanted tissue, organoid or cell therapy to function as a replacement for damaged native cells.
About iTolerance, Inc.
iTolerance is an early stage privately held regenerative medicine company developing technology to enable tissue, organoid or cell therapy without the need for life-long immunosuppression. Leveraging its proprietary biotechnology-derived Strepavidin-FasL fusion protein/biotin-PEG microgel (SA-FasL microgel) platform technology, iTOL-100, iTolerance is advancing a pipeline of programs using both allogenic pancreatic islets and stem cells that have the potential to cure diseases. The Company’s lead program, iTOL-101 is being developed for Type 1 Diabetes and in a pre-clinical non-human primate study, pancreatic islet cells co-implanted with iTOL-101 exhibited long-term function with control of blood glucose levels and restoration of insulin secretion without the use of chronic immune suppression. The Company’s second lead candidate, iTOL-102, is leveraging significant advancements in stem cells to derive pancreatic islets which allows an inexhaustible supply of insulin-producing cells. Utilizing iTOL-100 to induce local immune tolerance, iTOL-102 has the potential to be a cure for Type 1 Diabetes without the need for life-long immunosuppression. Additionally, the Company is developing iTOL-201 for liver failure and iTOL-301 as a potential regenerative protein and cell therapy that leverages stem cell sources to produce proteins or hormones in the body in conditions of high unmet need without the need for life-long immunosuppression. For more information, please visit itolerance.com.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the “safe-harbor” provisions of the Private Securities Litigation Reform Act of 1995. When used herein, words such as “anticipate”, “being”, “will”, “plan”, “may”, “continue”, and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking.
All forward-looking statements are based upon the Company’s current expectations and various assumptions. The Company believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. The Company may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various important factors, including, without limitation, anticipated levels of revenues, future national or regional economic and competitive conditions, and difficulties in developing the Company’s platform technology. Consequently, forward-looking statements should be regarded solely as the Company’s current plans, estimates and beliefs. Investors should not place undue reliance on forward-looking statements. The Company cannot guarantee future results, events, levels of activity, performance or achievements. The Company does not undertake and specifically declines any obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by law.
Investor Contact
Jenene Thomas
Chief Executive Officer
JTC Team, LLC
T: 833.475.8247
iTolerance@jtcir.com
[1] https://www.science.org/doi/abs/10.1126/sciadv.abm9881
SOURCE: iTolerance, Inc.
View source version on accesswire.com:
https://www.accesswire.com/701402/iTolerance-Inc-Pre-Clinical-Non-Human-Primate-Study-Demonstrates-Long-Term-Success-of-Allogeneic-Islet-Implantation-Without-Chronic-Immunosuppression-for-the-Treatment-of-Diabetes
