Entera Bio Announces Robust Pharmacokinetic Data for First-in-Class Oral GLP-2 Peptide Tablet Treatment for Patients with Short Bowel Syndrome

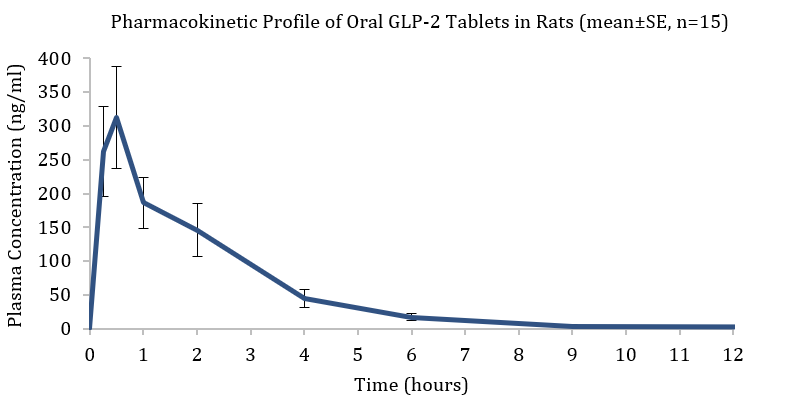
Pharmacokinetic Profile of Oral GLP-2 Tablets in Rats (mean±SE, n=15)
JERUSALEM, March 20, 2024 (GLOBE NEWSWIRE) — Entera Bio Ltd. (NASDAQ: ENTX), (“Entera” or the “Company”) a leader in the development of orally delivered peptides, announced today positive pharmacokinetic results from its collaborative research combining a proprietary long acting GLP-2 agonist developed by OPKO Health, Inc. (Nasdaq: “OPK”, or “OPKO”) with Entera’s proprietary N-Tab™ technology.
The program is focused on developing the first and only GLP-2 peptide tablet alternative for patients suffering from short bowel syndrome and additional disorders involving mucosal inflammation and nutrient malabsorption. Currently, the only approved GLP-2 agonist, which is marketed under the name Gattex® (teduglutide), requires daily sub-cutaneous injections. Zealand Pharma and Ironwood are developing long acting GLP-2 therapies requiring once and twice weekly injections.
Entera and OPKO completed a proof of concept (PoC) single dose pharmacokinetic study in rodents as the first validation for oral administration of the GLP-2 treatment. Oral tablets (1.8 mg; n=15) and IV injections (2 mg/kg; n=6) were administered to rats. Pharmacokinetic blood samples were taken for 24 hours post-dose and drug concentrations were analyzed by a validated LC-MS/MS method.
The study’s objectives were met with oral GLP-2 tablets exhibiting significant systemic exposure. Furthermore, plasma levels achieved with the oral tablet form of the GLP-2 analogue were about 10-fold higher than therapeutic plasma concentrations reported for subcutaneously administered teduglutide (Gattex® label). The pharmacokinetic analysis of the data obtained following the IV injections of the GLP-2 peptide showed the plasma half-life in rats to be about 6 times longer than the half-life reported for teduglutide in the same animal model. This data is consistent with previously reported PK data relating to OPKO’s GLP-2 peptide’s long acting profile, which had initially been developed as a weekly subcutaneous injection.
| Dose (mg) | Cmax (ng/ml) | Tmax (hr) | AUC1-24 (ng*hr/ml) |
| 1.8 | 312 | 0.5 | 678 |
“Given the challenging compliance rates attributed to injectable GLP-2 therapy, we believe a daily tablet format may address a significant unmet need in treating SBS patients more effectively. We are pleased with this first validation for the oral GLP-2 peptide program that we initiated in late 2023 in our collaboration with OPKO. The current PK data reinforces the data we previously published combining our N-Tab™ technology with teduglutide in tablet form1. Based on the promising results from the current PK study, we are planning to advance the program to assess pharmacologic effects in vivo. We look forward to updating on these data later in 2024,” said Miranda Toledano, Entera Chief Executive Officer.
About Short Bowel Syndrome
Short bowel syndrome (SBS) is a rare and potentially life-threatening malabsorptive condition caused by a significant loss of functional bowel mass (secondary to congenital defects or disease-associated loss of absorption) or physical bowel mass (secondary to extensive intestinal resection). SBS patients have a reduced ability to absorb nutrients and fluids and are at risk of malnutrition, unintended weight loss and additional symptoms due to the loss of essential vitamins and minerals2. SBS is the most common cause of chronic intestinal failure, accounting for approximately 75% of cases of chronic intestinal failure in adults and 50% such events in children.3
About Entera Bio
Entera is a clinical stage company focused on developing oral peptide or protein replacement therapies for significant unmet medical needs where an oral tablet form holds the potential to transform the standard of care. The Company leverages on a disruptive and proprietary technology platform and its pipeline includes five differentiated, first-in-class oral peptide programs, expected to enter the into the clinic (Phase 1 to Phase 3) by 2025. The Company’s most advanced product candidate, EB613 (oral PTH (1-34), teriparatide), is being developed as the first oral, osteoanabolic (bone building) once-daily tablet treatment for post-menopausal women with low BMD and high-risk osteoporosis, with no prior fracture. A placebo controlled, dose ranging Phase 2 study of EB613 tablets (n= 161) met primary (PD/bone turnover biomarker) and secondary endpoints (BMD). Entera is preparing to initiate a Phase 3 registrational study for EB613 pursuant to the FDA’s qualification of a quantitative BMD endpoint which is expected to occur in 2024. The EB612 program is being developed as the first oral PTH(1-34) tablet peptide replacement therapy for hypoparathyroidism. Entera is also developing the first oral oxyntomodulin, a dual targeted GLP1/glucagon peptide, in tablet form for the treatment of obesity; and first oral GLP-2 peptide tablet as an injection-free alternative for patients suffering from rare malabsorption conditions such as short bowel syndrome in collaboration with OPKO Health. For more information on Entera Bio, visit www.enterabio.com or follow us on LinkedIn, Twitter, Facebook, Instagram.
Cautionary Statement Regarding Forward Looking Statements
Various statements in this press release are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. All statements (other than statements of historical facts) in this press release regarding our prospects, plans, financial position, business strategy and expected financial and operational results may constitute forward-looking statements. Words such as, but not limited to, “anticipate,” “believe,” “can,” “could,” “expect,” “estimate,” “design,” “goal,” “intend,” “may,” “might,” “objective,” “plan,” “predict,” “project,” “target,” “likely,” “should,” “will,” and “would,” or the negative of these terms and similar expressions or words, identify forward-looking statements. Forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties. Forward-looking statements should not be read as a guarantee of future performance or results and may not be accurate indications of when such performance or results will be achieved.
Important factors that could cause actual results to differ materially from those reflected in Entera’s forward-looking statements include, among others: changes in the interpretation of clinical data; results of our clinical trials; the FDA’s interpretation and review of our results from and analysis of our clinical trials; unexpected changes in our ongoing and planned preclinical development and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates; the potential disruption and delay of manufacturing supply chains; loss of available workforce resources, either by Entera or its collaboration and laboratory partners; impacts to research and development or clinical activities that Entera may be contractually obligated to provide; overall regulatory timelines; the size and growth of the potential markets for our product candidates; the scope, progress and costs of developing Entera’s product candidates; Entera’s reliance on third parties to conduct its clinical trials; Entera’s expectations regarding licensing, business transactions and strategic collaborations; Entera’s operation as a development stage company with limited operating history; Entera’s ability to continue as a going concern absent access to sources of liquidity; Entera’s ability to obtain and maintain regulatory approval for any of its product candidates; Entera’s ability to comply with Nasdaq’s minimum listing standards and other matters related to compliance with the requirements of being a public company in the United States; Entera’s intellectual property position and its ability to protect its intellectual property; and other factors that are described in the “Cautionary Statements Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Entera’s most recent Annual Report on Form 10-K filed with the SEC, as well as the company’s subsequently filed Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. There can be no assurance that the actual results or developments anticipated by Entera will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Entera. Therefore, no assurance can be given that the outcomes stated or implied in such forward-looking statements and estimates will be achieved. Entera cautions investors not to rely on the forward-looking statements Entera makes in this press release. The information in this press release is provided only as of the date of this press release, and Entera undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except to the extent required by law.
1 Itin, C., Schwartz, P., Galitzer, H. et al. Oral Delivery Technology Enabling Gastro-Mucosal Absorption of Glucagon-Like-Peptide-2 Analog (Teduglutide) – A Novel Approach for Injection-Free Treatment of Short Bowel Syndrome. Int J Pept Res Ther 29, 59 (2023). https://doi.org/10.1007/s10989-023-10532-3
2 https://rarediseases.org/rare-diseases/short-bowel-syndrome/
3 Zhu C, Li Y. An updated overview of glucagon-like peptide-2 analog trophic therapy for short bowel syndrome in adults. J Int Med Res. 2022 Mar;50(3):3000605221086145. doi: 10.1177/03000605221086145. PMID: 35343263; PMCID: PMC8966062.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b328b568-7885-47c2-9cc8-c19a2d71be19
CONTACT: Contact: Entera Bio: Ms. Miranda Toledano Chief Executive Officer Entera Bio Email: miranda@enterabio.com

