PhaseV Launches New Version of its Machine Learning Platform, Facilitating Increased Adoption of Adaptive Clinical Trials

AdaptV Platform Optimizes Trial Design Across Teams With New Capabilities to Improve Group Sequential Design, Response Adaptive Randomization and BOIN I/II Designs
BOSTON, June 17, 2024 /PRNewswire/ — PhaseV, a pioneer in software and machine learning (ML) for adaptive clinical trial optimization, announced today the launch of a new upgraded version of its AdaptV platform for optimal design and closed-loop execution of adaptive clinical trials.
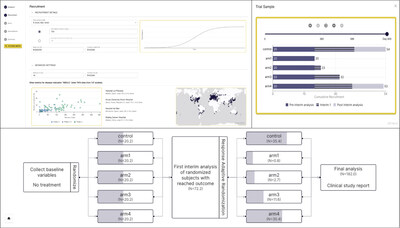
The AdaptV platform leverages an intuitive user interface and flexible software architecture along with proprietary algorithms, reinforcement learning, and causal ML to facilitate wider adoption of advanced and adaptive clinical trials. The updated platform will help to further reduce costs and shorten trial duration in order to benefit patients by maximizing efficiency and achieving higher trial success rates.
“Our platform enables clinical trial teams and strategic stakeholders to better understand the trade-offs of key decisions required throughout adaptive trial design and to make optimal decisions at any point during the trial,” said Raviv Pryluk, PhD, CEO and Co-founder of PhaseV. “By helping to overcome the inherent complexities, we unlock the tremendous potential of adaptive trials and facilitate increased adoption to support more efficient drug development and commercialization.”
Unveiled in 2023, PhaseV’s core technology has been adopted by dozens of pharma companies, CROs and biotech stakeholders across the U.S. and Europe.
The latest version of AdaptV includes an advanced mode to support more in-depth modifications of all relevant algorithmic and statistical parameters that go into trial design, accounting for the unique characteristics of a specific trial. It also incorporates a new proprietary algorithm for several types of adaptive trial designs:
- Group Sequential Design (GSD), an adaptive design that improves accuracy in small sample sizes and supports multi-arm trials while maintaining strict control for avoiding success declarations when the drug does not work (type-I error).
- Novel Response Adaptive Randomization (RAR) capabilities that support multi-interims in a trial while maintaining interactivity and accuracy at the highest standards.
- Bayesian Optimal Interval Phase I/II trial design (BOIN12) aligned with the FDA project Optimus, for optimization and dose selection for oncology drug development. The AdaptV platform can create BOIN12 designs in just minutes and facilitate real time adaptations required during trial execution.
About PhaseV
Leveraging the power of advanced causal inference and pushing the boundaries of ML, PhaseV detects hidden signals in clinical data and extracts actionable insights for planning the optimal next steps. The company’s technology enables optimal design and closed-loop execution of adaptive clinical trials, increasing efficiency and success rates. PhaseV is advancing paradigm shifts in the clinical trial world in order to bring new treatments to more patients, in a more precise and efficient way. Learn more at www.phaseVtrials.com and follow us on LinkedIn.
Media Contact:
Ellie Hanson
FINN Partners for PhaseV
ellie.hanson@finnpartners.com
929-588-2008
Photo: https://healthtechnologynet.com/wp-content/uploads/2024/06/PhaseV.jpg
Logo: https://healthtechnologynet.com/wp-content/uploads/2024/06/PhaseV_logo.jpg
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/phasev-launches-new-version-of-its-machine-learning-platform-facilitating-increased-adoption-of-adaptive-clinical-trials-302174059.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/phasev-launches-new-version-of-its-machine-learning-platform-facilitating-increased-adoption-of-adaptive-clinical-trials-302174059.html
SOURCE PhaseV