Autonomix Announces Positive Topline Results from First Five Lead-In Patients in Ongoing Human Clinical Trial

The first five patients successfully completed protocols with no immediate procedural-related complications or significant adverse events; Pain relief for responder group was experienced as quick as 1 day post-procedure
60% of subjects responded with a mean 6.33 reduction of pain on the VAS pain scale (from baseline of 8.0 to 1.67) at 7 days post-procedure
100% of this responder group had clinically meaningful pain relief at 7 days post-procedure
Responder patients reported a mean 78% improvement in quality of health and mean 45% improvement in quality of life at 7 days post-procedure
Trial remains on track to complete enrollment by year-end 2024
Management to host live webcast to discuss results today, June 18th at 8:30 a.m. ET
THE WOODLANDS, TX, June 18, 2024 (GLOBE NEWSWIRE) — Autonomix Medical, Inc. (NASDAQ: AMIX) (“Autonomix” or the “Company”), a medical device company focused on advancing innovative technologies to revolutionize how diseases involving the nervous system are diagnosed and treated, today announced preliminary positive results from the first five “lead-in” patients in the Company’s ongoing proof-of-concept (PoC) human clinical trial evaluating the safety and effectiveness of delivering transvascular energy to ablate relevant problematic nerves and mitigate pain in patients with pancreatic cancer pain. The goal of this trial is to assess pain reduction via radiofrequency (RF) ablation. The Company’s catheter-based microchip sensing array used to detect and differentiate neural signaling was not used in this trial and will be evaluated in future studies. As previously announced, management will host a webcast presentation to discuss the preliminary results today, Tuesday, June 18, 2024, at 8:30 a.m. ET (details below).
The first five patients were enrolled and treated according to protocol in the beginning of the trial to familiarize the Principal Investigator (PI) with the procedure and will not be included in the analysis of the trial objectives. These first five “lead-in” patients successfully completed the procedure per protocol with no immediate procedural-related complications or significant adverse events.
“We are very pleased with these initial positive results, which confirm our ability to treat this historically difficult-to-treat cancer pain with catheter-based transvascular RF ablation. While preliminary, we are highly encouraged with the level of pain reduction and quality-of-life improvement these patients gained and we hope continue throughout the remainder of the trial. While these data will not be included in the final analysis, they provided our clinical team valuable insight into optimizing the procedure, particularly the catheter entry point, where 3 of the 5 patients who saw a pain response had femoral access of the catheter and the 2 that did not respond had brachial access. We believe access factors played a role in the ability to ablate the appropriate nerves to see mindful pain reduction in the brachial access patients, as the bias of the catheter appears less optimal in ablating the target nerves from this approach to the target anatomy,” commented Dr. Robert Schwartz, Co-Founder and Chief Medical Officer of Autonomix.
The primary objective of the PoC human clinical trial is to assess the success rate of ablating relevant nerves to mitigate pain in patients with pancreatic cancer pain utilizing RF ablation in a transvascular approach to the nerves in the region. Secondary objectives include assessing the incidence of device- and procedure-related adverse events up to 4-6 weeks post-procedure; estimating the change in pain levels from pre- to post-procedure; and estimating the change in quality of life from pre- to post-procedure. All subjects who have had a successful procedure were evaluated at 7 days, 4–6 weeks, and at 3 months post-procedure. All patients entered the study with severe abdominal pain from unresectable pancreatic cancer and a life expectancy of 3 months or less. Following the successful completion of the procedure, two subjects have since succumbed to their disease. Both events were expected outcomes and not related to the trial procedure.
“We are very encouraged by the results from the first five lead-in patients from our ongoing first-in-human trial and while they appear to support our ability to have a meaningful impact on pain via RF ablation when we successfully ablate targeted nerves, it is only part of the story. We believe that in future studies and with our catheter-based microchip sensing array, which we expect to be able to detect and target overactive nerves causing pain and confirm nerve death after ablation, we may be able to improve on the level of responders. We are extraordinarily pleased with the positive outcome this procedure is having on responding patients and the relief they are experiencing from the difficulty of end-stage pancreatic cancer,” Lori Bisson, Executive Vice Chairman of Autonomix added.
Click here to watch patient testimonials from some of the trial participants and treating physician.
Summary of Topline Results 7 Days Post-Procedure
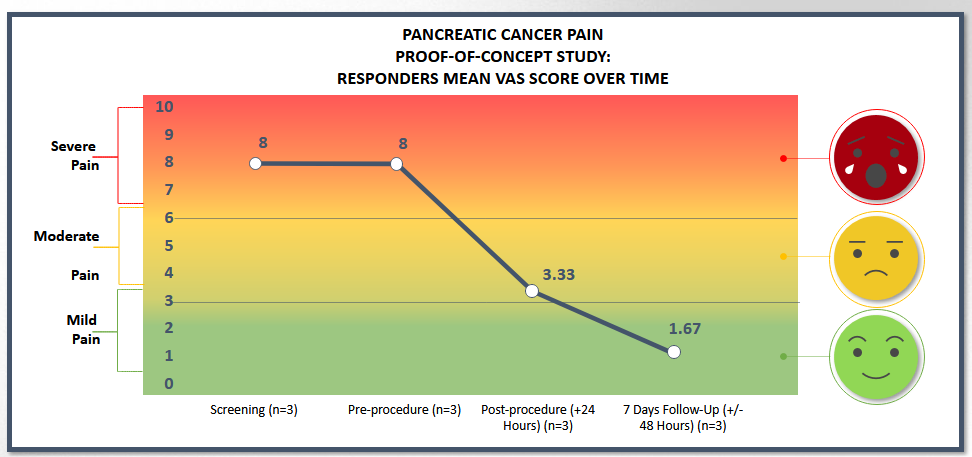
- 24 hours post-procedure showed an overall mean VAS Pain Score of 2.3 after treating each of the five subjects.
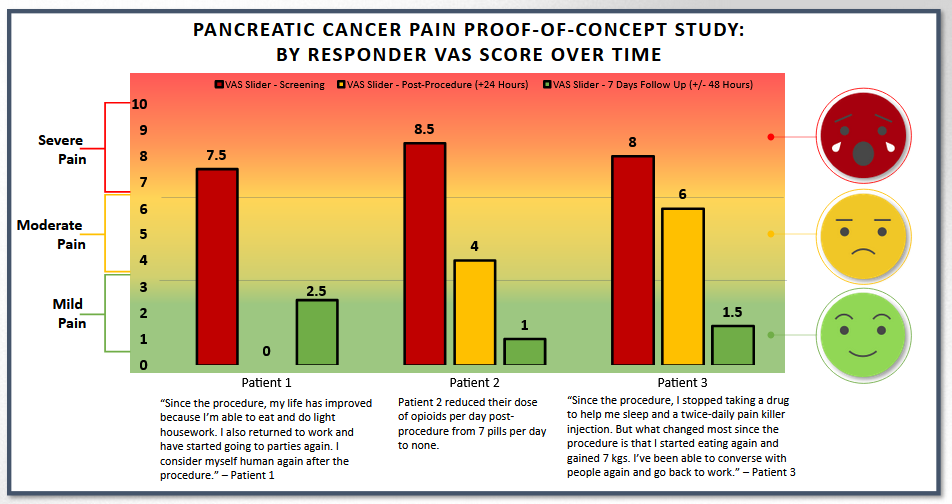
- Three patients were treated with femoral access and two were treated with brachial access. Patients treated with brachial access showed no improvement in their pain scores (or worsened) while all patients treated with femoral access positively responded to treatment. The results presented in the charts above are for the three patients in the responder group.
- 60% of subjects responded with a mean 6.33 reduction of pain on the VAS pain scale (from baseline of 8.0 to 1.67) at 7 days post-procedure.
- Pain relief was experienced as quick as 1-day post-procedure for the three patients in the responder group.
- Reduction of pain score occurred simultaneously as the subject’s underlying disease (pancreatic cancer tumor) continued to grow. This is beneficial for patients who are considered end of life given their advanced stage of disease.
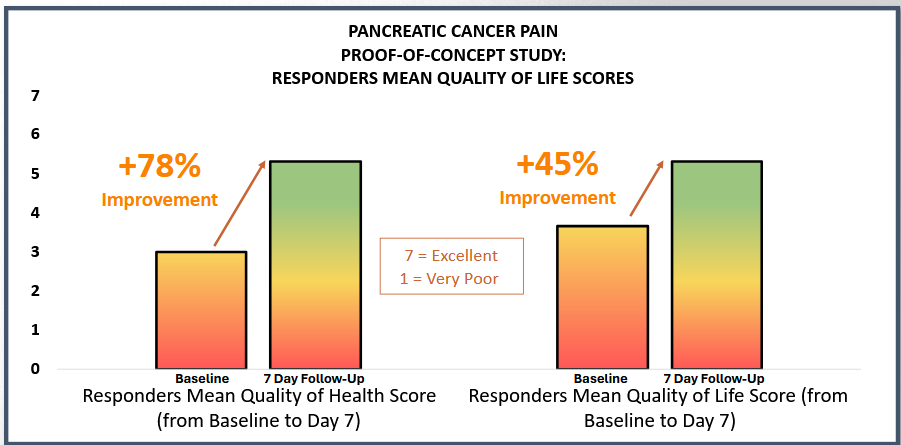
- Responder patients reported a mean 78% improvement in quality of health at 7 days.
- Responder patients reported a mean 45% improvement in quality of life at 7 days.
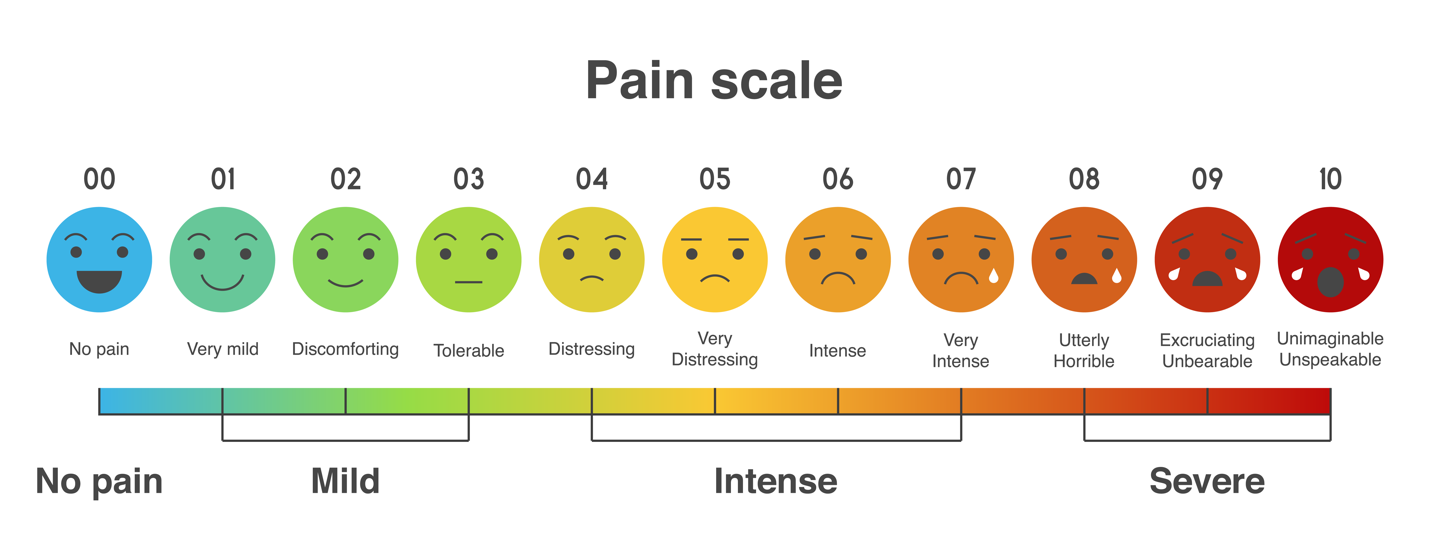
When evaluating the total treated population, including non-responders, the mean reduction in the VAS pain score was a 2.9 point reduction, or 43%, in pain scores reported pre-procedure to day 7.
As previously announced, Autonomix has amended the trial protocol to include the gathering of additional information on tumor encroachment on the vessels as well as other key bio-measurements that may correlate with effective nerve ablation. Additionally, the Company has further defined severe pain for inclusion criteria as a 7 or above on the VAS scale as indicated by the patient rather than physician determination. A total of twenty (20) additional subjects will be enrolled in the trial that will be formally included in the trial data results and analysis of trial objectives. Suitability is determined by the primary oncologist caring for the patients with the treating Principal Investigator confirming eligibility for the trial. Autonomix commenced patient screening under the amended protocol in May 2024 and remains on track to complete enrollment in the PoC human clinical trial by year-end.
The Company’s catheter-based technology is being developed to do two things: sense neural signals associated with pain or disease and deliver targeted ablation to those nerves for treatment. Autonomix believes this technology is a better alternative to the current approaches commonly used today, where doctors either rely on systemic drugs like opioids that lose effectiveness and have unwanted side effects or treat suspected areas blindly in hopes of hitting the right nerves, an approach that is often inaccurate and can miss the target and even cause collateral damage to surrounding parts of the body.
The Company is initially developing its technology to address pancreatic cancer-related pain, with plans for follow-on indications pending the results of the initial data. Current approaches, primarily relying on opioids or invasive ethanol injections, can provide only limited relief and may lead to risky side effects. For more information about the Company’s technology, please visit autonomix.com.
Webcast Presentation Details
As previously announced, Autonomix management will host a webcast presentation for investors, analysts, and other interested parties today, Tuesday, June 18, 2024 at 8:30 a.m. ET to discuss the preliminary results. Interested participants may register for the event here. The live webcast will be accessible on the Events page of the Investors section of the Autonomix website, autonomix.com, and will be archived for 90 days.
About Autonomix Medical, Inc.
Autonomix is a medical device company focused on advancing innovative technologies to revolutionize how diseases involving the nervous system are diagnosed and treated. The Company’s first-in-class technology platform includes a catheter-based microchip sensing array that has the ability to detect and differentiate neural signals with approximately 3,000 times greater sensitivity than currently available technologies. We believe this will enable, for the first time ever, transvascular diagnosis and treatment of diseases involving the peripheral nervous system virtually anywhere in the body.
We are initially developing technology for the treatment of pain, with initial trials focused on pancreatic cancer, a condition that causes debilitating pain and is without an effective solution. Our technology constitutes a platform to address dozens of indications, including cardiology, hypertension and chronic pain management, across a wide disease spectrum. Our technology is investigational and has not yet been cleared for marketing in the US.
For more information, visit autonomix.com and connect with the Company on X, LinkedIn, Instagram and Facebook.
Forward Looking Statements
Some of the statements in this release are “forward-looking statements,” which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the potential of the technology to treat pain associated with pancreatic cancer, to successfully enroll patients within the specific timeframe, and to complete its clinical study in pancreatic cancer pain. Such forward-looking statements can be identified by the use of words such as “should,” “might,” “may,” “intends,” “anticipates,” “believes,” “estimates,” “projects,” “forecasts,” “expects,” “plans,” and “proposes.”
Although Autonomix believes that the expectations reflected in these forward-looking statements are based on reasonable assumptions, there are a number of risks and uncertainties that could cause actual results to differ materially from such forward-looking statements. You are urged to carefully review and consider any cautionary statements and other disclosures, including the statements made under the heading “Risk Factors” and elsewhere in the Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (SEC) on May 31, 2024. Forward-looking statements speak only as of the date of the document in which they are contained and Autonomix does not undertake any duty to update any forward-looking statements except as may be required by law.
Investor and Media Contact
JTC Team, LLC
Jenene Thomas
833-475-8247
autonomix@jtcir.com

