Rutherrin(R) Increases Efficacy of Immunotherapy Preclinically

TORONTO, ON / ACCESSWIRE / June 18, 2024 / Theralase® Technologies Inc. (“Theralase®” or the “Company“) (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that in preclinical research, it’s lead drug formulation, Rutherrin®, has demonstrated an ability to increase the efficacy of immunotherapy.
Immunotherapy, the latest technology in the war on cancer, can come in various forms; including: checkpoint inhibitors, Chimeric Antigen Receptor (“CAR“) T-Cell therapy, cytokines, immunomodulators, cancer vaccines, monoclonal antibodies and oncolytic viruses, but the fundamental Mechanism Of Action (“MOA“) of all of these immunogenic drugs is to stimulate the immune system to destroy cancer cells.
Cancer cells hide from the immune system by overexpressing proteins on their cellular surface, known as checkpoint proteins, that prevent the immune system from recognizing and subsequently destroying them. They thus remain incognito to the one failsafe that can protect the human body, the immune system.
The MOA of checkpoint inhibitors is to block the PD-L1 (checkpoint protein) on the cancer cell surface, allowing the immune system to detect and destroy the cancer cell; however, resistance to immunotherapy remains one of the major challenges in this form of treatment. In an attempt to overcome this resistance, multiple immunotherapy treatments are delivered to the patient, which may ultimately lead to a diminishing return in efficacy and a corresponding increase in patient serious adverse events and even treatment-related death.
Theralase®’s latest research demonstrates that Rutherrin® enhances the MOA of immunotherapy by not only killing cancer cells directly, but also significantly reducing the amount of PD-L1 proteins expressed by cancer cells; hence, reducing the number of target checkpoint proteins that need to be blocked by checkpoint inhibitors.
This results in an elegant one-two-three punch on the destruction of cancer cells; where, Rutherrin® delivers the first punch, targeting and destroying cancer cells directly, as well as the second punch, by reducing the number of PD-L1 proteins expressed. This allows immunotherapeutic drugs to deliver the third and final punch, blocking the PD-L1 proteins remaining, allowing the immune system to significantly increase their recognition of cancer cells and hence their destruction. As a result, this technological advance increases both the safety and efficacy of immunotherapy, as less treatments would be required to induce the same clinical effect.
As shown in Figure 1, treatment of human cancer cells; specifically, Non-Muscle Invasive Bladder Cancer (“NMIBC“) and Glio Blastoma Multiforme (“GBM“) with Rutherrin® significantly reduced the expression of PD-L1 checkpoint proteins on the surface of the cancer cells; hence, allowing immunogenic drugs a greater opportunity to block those remaining. This would allow the immune system a much better opportunity to identify them and target them for destruction.
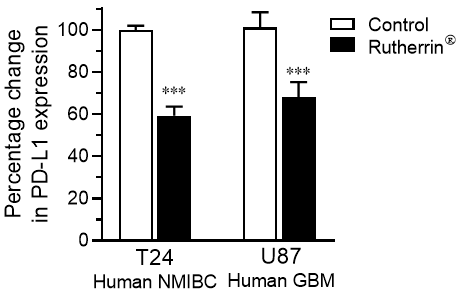
As a primary MOA, Rutherrin®, has been demonstrated clinically to destroy NMIBC, when activated by light, and preclinically to destroy GBM and Non-Small Cell Lung Cancer (“NSCLC“), when activated by x-ray radiation.
As a secondary MOA, Rutherrin®, has been demonstrated preclinically to unmask cancer cells through dual immunogenic check points; specifically, CD47 (previously reported by Theralase®) and now PD-L1 inhibition. This down regulation of immunogenic check points allows the cancer cell to be detected and destroyed by the immune system, resulting in a process known as Immunogenic Cell Death (“ICD“). ICD is characterized by the secretion of Damage-Associated Molecular Patterns (“DAMPs“), which are transported to the cell surface during ICD.
Calreticulin (“CRT“), one of the DAMPs found in the lumen of the endoplasmic reticulum, is translocated to the surface of dying cells, after the induction of ICD, where it functions as an “eat me” signal for the immune system.
As shown in Figure 2, radiation alone (Control) causes a mild increase in the percentage of cells expressing CRT on their surface, while the combined treatment of Rutherrin®, plus x-ray radiation significantly increases the surface expression of CRT. The activation of the immune system, due to increased CRT expression, introduces new opportunities for higher cancer cell kill, both locally and against distant/metastatic tumours.
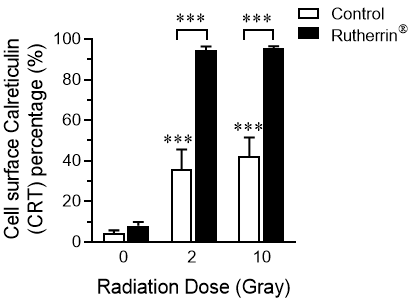
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated, “It is one of humanity’s greatest health challenges in the 21st century, that cancer of various forms, affects and kills millions of people each and every year, without discrimination. Immunotherapy is the latest technology that attempts to harness the power of the immune system to detect and destroy cancer cells; however, these same cancer cells, treated with immunotherapy, develop mechanisms to avoid detection by the immune system, which consequently has an adverse effect on how effectively they react to therapy. In that regard, in a clinical setting, the term “resistance to immunotherapy” is applied; primary resistance denoting a failure to respond to the treatment from day one, while secondary resistance denoting a relapse following the initial response to immunotherapy. Despite remarkable scientific progress in this field, attempts to develop new strategies against cancer resistance to immunotherapy have proven difficult. We are very pleased with the results of our latest research, demonstrating that our lead drug formulation, Rutherrin®, is able to maintain a therapeutic balance between various “accelerators” and “brakes” of our immune system to ensure that it is sufficiently engaged in attack against malignant cells, while avoiding destruction of healthy cells and tissues.“
Roger DuMoulin-White, B.E.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated, “All cancer therapies, in their essence, attempt to destroy the disease, with minimal to no side effects and allow the patient a complete response with no recurrence; however, despite recent advances in the treatment of specific cancer types, many patients still struggle to respond to cancer treatments and are left with significant side effects. The PD-L1 (immune checkpoint protein) functions as a “brake” on the innate immune system, while Calreticulin (“eat me” signal) functions as an accelerator. We are very pleased to demonstrate that Rutherrin ® has the potential of releasing the “brake” and applying the “accelerator” to the immune system at the right time and thereby unleashing the power of our immune system to attack and destroy cancerous cells, while sparing healthy ones. By stimulating the inherent ability of our immune system to protect and defend our bodies against cancer, Rutherrin® has the potential to establish an entirely new paradigm for cancer therapy.”
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains “forward-looking statements” within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company’s proposed development plans with respect to small molecules and their drug formulations. Forward looking statements may be identified by the use of the words “may, “should“, “will“, “anticipates“, “believes“, “plans“, “expects“, “estimate“, “potential for” and similar expressions; including, statements related to the current expectations of Company’s management for future research, development and commercialization of the Company’s small molecules and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations, the risk that access to sufficient capital to fund the Company’s operations may not be available on terms that are commercially favorable to the Company or at all, the risk that the Company’s small molecule and drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company’s fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company’s ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company’s ability to control or predict.
Readers should not unduly rely on these forward-looking statements, which are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate as such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For investor information on the Company, please feel to reach out Investor Inquiries – Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
X 224
khachey@theralase.com
SOURCE: Theralase Technologies Inc.
View the original press release on accesswire.com
View the original press release on accesswire.com
