Clinical study showing ClarityDX Prostate accurately predicts a patient’s risk of having clinically significant prostate cancer published in Nature Digital Medicine

- Results of an international multi-center study to evaluate the accuracy of the ClarityDX Prostate screening test are now available to the public
- Open access article published in npj Digital Medicine. Click the link for full-text article: https://rdcu.be/dLqzD
EDMONTON, AB, June 26, 2024 /PRNewswire/ – Nanostics Inc., a precision health company developing diagnostic tests with its ClarityDX® platform technology, is thrilled to announce the publication of the research supporting the development of the ClarityDX Prostate test. This test combines an optimized machine learning platform with blood-based biomarkers for clinically significant prostate cancer and clinical biomarker data to generate the patient’s risk score for clinically significant (defined as Grade Group 2 and above) prostate cancer.
“Now that the results have been published in Nature Digital Medicine, scientists, physicians and patients can all evaluate the scientific evidence for the performance of ClarityDX Prostate as an adjunctive screening tool for prostate cancer,” said Dr. John Lewis, CEO of Nanostics and Bird Dogs Chair of Translational Oncology at the University of Alberta.
Early detection and treatment of clinically significant prostate cancer is key to survivability. Currently, men are screened for prostate cancer using the PSA test. However, using the PSA test alone could result in many men undergoing prostate biopsies when they have no, or low-risk prostate cancer, and undergoing unnecessary treatments when they have low-risk cancer. North American and European urology associations now recommend that men with elevated PSA levels use adjunctive tests to better inform their decision to biopsy or not.
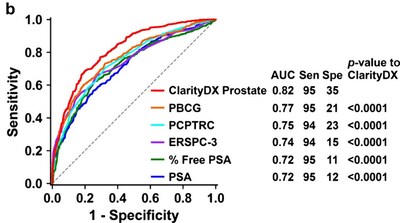
According to the 3448-patient study titled, “Development of an effective predictive screening tool for prostate cancer using the ClarityDX machine learning platform“, validation of ClarityDX Prostate showed it to have 95% sensitivity, 35% specificity, 54% positive predictive value, and 91% negative predictive value for predicting clinically significant prostate cancer. Using ClarityDX Prostate could avoid up to 35% of unnecessary prostate biopsies.
The study also compared ClarityDX Prostate to other model-based risk calculators and PSA alone showing that ClarityDX Prostate was three times more accurate at predicting the risk of clinically significant prostate cancer. The study supports the use of ClarityDX Prostate as an adjunctive test for men with elevated PSA levels to help patients and their healthcare providers decide if a prostate biopsy is necessary.
“This study shows that ClarityDX Prostate is accurate at predicting if the patient has grade group 2 and above prostate cancer,” said Dr. Eric Hyndman, Urologist and Chief Medical Officer at Nanostics. “Using this as an adjunctive test after PSA screening will give physicians more information to help decide if their patient should undergo a prostate biopsy or not.”
This study was supported by funding from the Alberta Cancer Foundation, Bird Dogs, NRC-IRAP, Alberta Innovates, Ride for Dad and the Prostate Cancer Fight Foundation, and Prostate Cancer Canada.
Go to www.nanosticsdx.com for more information and to order ClarityDX Prostate. Please direct all inquiries about the ClarityDX Prostate test to Nanostics via email at info@nanosticsdx.com or telephone 1-800-672-2027.
About Nanostics Inc.
Nanostics is a private Canadian company that develops and commercializes novel and noninvasive diagnostic tests. Its core technology, ClarityDX®, uses advanced machine learning algorithms to create a disease risk score. ClarityDX is applicable to a wide range of cancers and other diseases. Nanostics’ lead product, ClarityDX Prostate®, is a test that improves the accuracy of detecting clinically significant prostate cancer. Read more at: www.nanosticsdx.com. Follow Nanostics on LinkedIn, YouTube, Facebook, and Instagram.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/clinical-study-showing-claritydx-prostate-accurately-predicts-a-patients-risk-of-having-clinically-significant-prostate-cancer-published-in-nature-digital-medicine-302183294.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/clinical-study-showing-claritydx-prostate-accurately-predicts-a-patients-risk-of-having-clinically-significant-prostate-cancer-published-in-nature-digital-medicine-302183294.html
SOURCE Nanostics