Sona’s Cancer Therapy Triggers Abscopal Effect, Eliminating Distant Tumors In Preclinical Melanoma Study

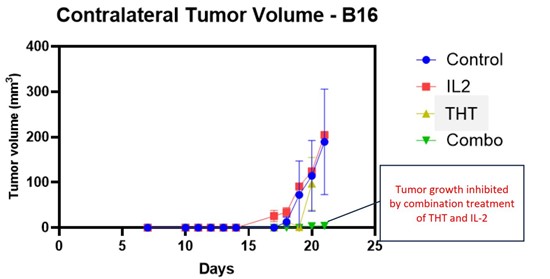
Halifax, Nova Scotia–(Newsfile Corp. – June 26, 2024) – Sona Nanotech Inc. (CSE: SONA) (OTCQB: SNANF) (the “Company” or “Sona”) is pleased to announce that a detailed biomarker analysis of the recently reported pre-clinical melanoma study conducted at Dalhousie University (the “Study”) indicates that, beyond shrinking tumors on its own, Sona’s Targeted Hyperthermia Therapy (“THT”) also stimulates the innate immune system to target and eliminate untreated (contralateral) tumors when combined with a standard immunotherapeutic drug, IL-2. (See figure #1, below.) On the basis of efficacy data achieved in two different murine cancer models (triple negative breast cancer and malignant melanoma), the Company is now proceeding with safety and biocompatibility testing that will be required by regulating agencies to enter into human studies.
Sona’s Chief Medical Officer and the Study’s principal investigator, Dr. Carman Giacomantonio, commented, “With two separate murine cancer models completed, we now believe it is clear that Sona’s THT therapy causes cancer specific proteins (cancer antigens) to become visible to the immune system. This in turn causes novel, innate immune responses ultimately enabling development of cancer-specific immunity. This is essentially the goal of all current immunotherapy research and treatment strategies. When combined with a standard of care immunotherapeutic drug (IL-2), the resultant immunity in our models was strong enough to generate an immune response in remote (contralateral) tumors. Finally, we were excited to observe that the immunity generated by Sona’s gold nanorod-based THT therapy in our preclinical models is lasting. In other words, we observed a “vaccine effect” whereby we were unable to immediately grow new tumors in mice whose primary tumors had responded to Sona’s gold nanorod THT therapy combined with standard immunotherapy. This is the proof of concept we were looking for.”
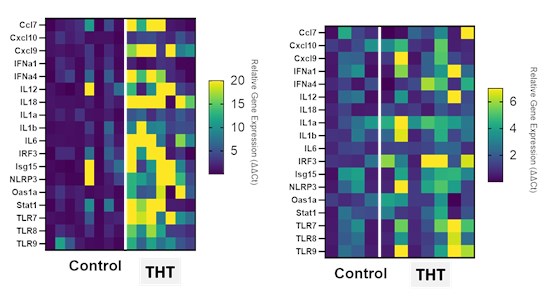
Following treatment with Sona’s THT for melanoma in mice, an analysis of key metrics of immune memory showed that remote tumors could not be established in mice with primary tumors previously treated with Sona’s gold nanorod-based THT therapy when combined with standard (IL-2) immunotherapy. Furthermore, an examination of the upregulation of inflammatory gene expression for 17 genes for both Sona’s 4T1 (triple negative breast cancer) and B16 (melanoma) models showed a strong pattern of expression enhancement, a further indication of the success Sona’s THT therapy had in effectuating a fundamental change to the innate immune system. (See figure #2, below.)
Sona CEO, David Regan, commented, “Seeing the gene expression data which supports the longevity of the new immunity achieved together with the observation that new cancer tumors did not take in the treated mice in this preclinical study, gives hope that Sona’s therapy could be used with immunotherapeutic drugs to treat cancer effectively and reduce the likelihood of recurrences. Given our success in demonstrating this concept in two different murine cancer models, we are now moving forward aggressively with the study program required by regulators that would allow us to progress this novel therapy into the clinic for a first-in-human trial. Our initial indication will target the thousands of people who suffer from late-stage, unresectable melanoma for which no other therapy has worked.“
The results discussed in this release are preliminary and have not been subject to peer review. Upon completion, the Company expects that the full Study will be submitted for peer review and scientific journal publication.
Figure 1: Sona’s THT Therapy Combined with IL-2 Inhibits Growth of Distant Tumors
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/5500/214441_sonafig1nocaption%20(1).jpg
4T1 (Triple Negative Breast Cancer) B16 (Melanoma)
Figure 2: Gene Expression Heat Maps
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/5500/214441_4beae7dd94ed7a13_003full.jpg
Contact:
David Regan, CEO
+1-902-536-1932
david@sonanano.com
About Sona Nanotech Inc.
Sona Nanotech, a nanotechnology Life Sciences company, is developing Targeted Hyperthermia Therapy™, a photothermal cancer therapy, which uses therapeutic heat to treat solid cancer tumors. The heat is delivered to tumors by infrared light that is absorbed by Sona’s gold nanorods in the tumor and re-emitted as heat. Therapeutic heat (41-48°C) stimulates the immune system, shrinks tumors, inactivates cancer stem cells, and increases tumor perfusion – thus enabling drugs to reach all tumor compartments more effectively. The size, shape, and surface chemistry of the gold nanorods target the leaky vasculature of solid tumors, and the selective thermal sensitivity of tumor tissue enables the therapy to deliver clean margins. Targeted Hyperthermia Therapy promises to be safe, effective, minimally invasive, competitive in cost, and a valuable adjunct to drug therapy and other cancer treatments.
Sona has developed multiple proprietary methods for the manufacture of gold nanoparticles which it uses for the development of both cancer therapies and diagnostic testing platforms. Sona Nanotech’s gold nanorod particles are cetyltrimethylammonium (“CTAB”) free, eliminating the toxicity risks associated with the use of other gold nanorod technologies in medical applications with an assessment by the Nanotechnology Characterisation Laboratory that detected neither endotoxins nor microbial contamination. It is expected that Sona’s gold nanotechnologies may be adapted for use in applications, as a safe and effective delivery system for multiple medical treatments, subject to the approval of various regulatory boards, including Health Canada and the FDA.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION: This press release includes certain “forward-looking statements” under applicable Canadian securities legislation, including statements regarding the anticipated applications and potential opportunities of Targeted Hyperthermia Therapy, Sona’s preclinical and clinical study plans and its product development plans. Forward-looking statements are necessarily based upon a number of assumptions or estimates that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements, including the risk that Sona may not be able to successfully obtain sufficient clinical and other data to submit regulatory submissions, raise sufficient additional capital, secure patents or develop the envisioned therapy, and the risk that THT may not prove to have the benefits currently anticipated. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking statements. Sona disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Not for distribution to United States newswire services or for dissemination in the United States

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/214441