Trethera Receives $2M NIH Grant for Preclinical Development in the Pediatric Neurologic Disease Acute Disseminated Encephalomyelitis

LOS ANGELES, July 16, 2024 (GLOBE NEWSWIRE) — Trethera Corporation, a clinical stage biopharmaceutical company committed to developing novel drugs targeting nucleotide metabolism for the treatment of cancer and autoimmune diseases, announced today that it was awarded a $2 million NIH Small Business Innovation Research (SBIR) grant for preclinical studies treating acute disseminated encephalomyelitis (ADEM), a neurologic disease principally of children. Trethera’s clinical stage and first-in-class drug, TRE-515, holds the only FDA Orphan Drug designation for ADEM.
ADEM is an autoimmune disease that can present with fever and difficulty walking as well as loss of consciousness and coma. Viral infections, such as influenza, mumps, or COVID, frequently precede the disease. ADEM is a rare disease, affecting 12,000 to 15,000 patients a year in the United States, with most cases occurring in 6 to 8 year-old children. The grant will advance preclinical studies to enable clinic entry for a disease where no approved therapies currently exist.
TRE-515 is the only drug to receive an FDA Orphan Drug designation for the treatment of ADEM, a designation that confers substantial advantages, including FDA assistance in designing clinical trials, access to the FDA Orphan Drug Grants Program, exemption from the $4M drug approval application fee, and eligibility for seven years of marketing exclusivity. Furthermore, should the FDA approve TRE-515 for commercial use in ADEM, Trethera would be eligible for a pediatric priority review voucher. Combining the study data generated through this grant and the Orphan Drug designation, Trethera plans to collaborate with the FDA to define the most expedient clinical trial path to commercialization.
“Achieving both NIH funding and FDA Orphan Drug designation confers multiple external validations of our potential to treat ADEM,” said Dr. Ken Schultz, principal investigator and Trethera CEO. “Our team is highly motivated by the potential to develop the first FDA approved treatment to save children’s lives and improve outcomes in ADEM. Our strategy of combining TRE-515 commercial development for a rare disease with ongoing efforts to treat more common diseases, such as solid tumors and Crohn’s, provides multiple FDA approval opportunities, thereby reducing overall development risk.”
“Approximately half of ADEM patients recover with hospitalized intensive care. For others, ADEM can be fatal or lead to lifelong disability. Any drug that could improve these outcomes for patients would be groundbreaking. TRE-515 could significantly benefit ADEM patients beyond the available therapeutic options,” said Trethera Scientific Advisory Board member Dr. Larry Steinman. Dr. Steinman is a distinguished immunologist and pediatric neurologist at Stanford University.
“The pathology of ADEM involves a severe bout of inflammation in the central nervous system that can include the brain, spinal cord, and sometimes the optic nerves. The inflammation damages myelin, the protective substance that coats nerve fibers throughout the central nervous system. No medications have been specifically approved by the FDA to treat ADEM,” said UCLA’s Dr. Peter Clark, member of the Trethera Scientific Advisory Board and grant co-investigator.
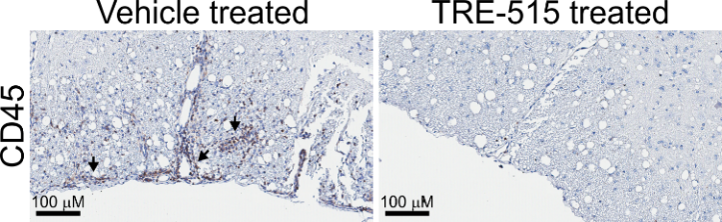
Figure 1: Representative stained spinal cord sections from a mouse ADEM model. Arrows point to regions of leukocyte infiltration.
A discussion summary from the independent panel of NIH scientists and physicians that reviewed Trethera’s proposal noted the “strong scientific premise and rigor…. established safety in Phase I clinical trial for solid tumors… and commercialization potential is high.”
Harvard’s Research Director of Neuroimmunology and Trethera Scientific Advisory Board member, Dr. Michael Levy, noted “The clinical treatment for an ADEM patient remains complex on multiple levels. Treating a disease with a real mortality risk in a vulnerable pediatric population creates a challenging role for the attending physician especially given the lack of FDA approved therapies. I look forward to the day TRE-515 can be FDA approved to provide confidence to ADEM patients and their parents.”
Sources: J Neurol. 2020 Oct;267(10); Int Care Med. 2008 Mar;34(3); Neuro. 2001 May 22;56(10)
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera’s innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule twice designated by the FDA as an Orphan Drug. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as “may,” “might,” “will,” “will likely result,” “would,” “should,” “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “continue,” “target” or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
The content of this press release is solely the views of its authors and does not represent the official views of the NIH.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/bfbdd798-3243-4875-addb-25512db370d7

