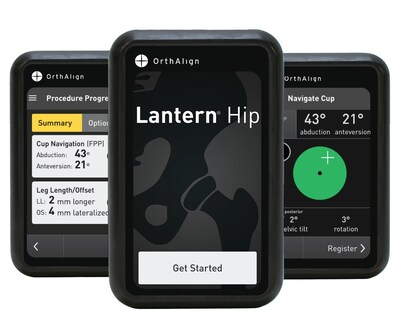
OrthAlign Receives FDA 510(k) Clearance for Lantern® Hip

IRVINE, Calif., Oct. 3, 2024 /PRNewswire/ — OrthAlign, Inc., a privately held medical device company, announces FDA 510(k) clearance of their Lantern Hip handheld technology for direct anterior total hip arthroplasty with the patient in the supine position. Lantern Hip is the latest addition to the Lantern platform, joining existing applications for total knee, revision knee, and partial knee arthroplasty.
Lantern Hip builds upon the legacy of HipAlign® with enhanced technology and usability. Next-generation accelerometers and gyroscopes offer orthopedic surgeons a streamlined workflow, real-time navigation for cup positioning, and restoration of leg length and offset. The technology enables individualized cup positioning compared across multiple planes, including the functional pelvic plane, anterior pelvic plane, and coronal plane, with live pelvic tracking. Designed for personalized patient care, Lantern Hip eliminates the need for pre-operative imaging or capital equipment and is compatible with most implant systems, making it an ideal choice for the ASC setting.
“Technologies like Lantern Hip represent the future of orthopedic surgery,” said Edwin Su, MD, orthopedic surgeon at the Hospital for Special Surgery in New York, NY. “The system provides the information I need to accurately position each patient’s hip components based on their unique anatomy, and I can hit the leg length and offset targets I want to achieve. These advancements, coupled with the new ability to place the cup in relation to the functional pelvic plane, hold the potential to greatly improve patient outcomes.”
“Adding the total hip application to the Lantern platform is an enhancement that not only broadens our capabilities, but also reinforces our commitment to providing innovative solutions,” said Eric Timko, Chairman and CEO of OrthAlign. “As technology becomes the standard of care in total joint replacement, it’s essential to deliver solutions that are clinically, operationally, and economically efficient. Lantern Knee and Balance have been a great success for OrthAlign and now, with Lantern Hip, surgeons can confidently and easily take technology to any site of service for their total hip replacements. I applaud our internal team and surgeon advisors for the countless hours spent developing a product that we expect will make a positive impact on healthcare.”
Lantern Hip will be on display at the American Association of Hip and Knee Surgeons (AAHKS) annual meeting in Grapevine, TX in November. To learn more, contact info@orthalign.com.
About OrthAlign, Inc.
OrthAlign is a medical device company with a focus on delivering practical, cutting-edge technologies for orthopedic surgery. With a commitment to innovation and excellence, OrthAlign provides surgeons with user-friendly, cost-effective solutions to help improve patient care in joint replacement. In 2023, the company celebrated a record-breaking year with over $50 million in global revenue, reflecting its dedication to growth and leadership in the industry.
Driven by the belief that everyone deserves exceptional healthcare, we are committed to making empowering technologies accessible to all.
For more information regarding OrthAlign, please visit www.orthalign.com.
HIPALIGN®, LANTERN®, and ORTHALIGN® are registered trademarks of OrthAlign, Inc.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/orthalign-receives-fda-510k-clearance-for-lantern-hip-302267260.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/orthalign-receives-fda-510k-clearance-for-lantern-hip-302267260.html
SOURCE OrthAlign