The Exosome Production Method Patent by Panacell Biotech Registered as Original Technology

SEOUL, South Korea, Oct. 3, 2024 /PRNewswire/ — Panacell Biotech Co., Ltd. (Panacell Biotech) announced that the patent related to their exosome manufacturing method filed last year was registered as a core technology in the patent registry on August 7, 2024, following the Patent Act. Panacell Biotech, whose Chief Executive Officer (CEO) is Dr. Seung Ho Choi, is a research institute specializing in stem cells and a cell processing institution certified by the Ministry of Food and Drug Safety.
Panacell Biotech has filed three patents on cosmetic compositions containing exosomes produced using established methods for manufacturing exosomes derived from natural killer (NK) cell culture supernatant, adipose-derived stem cell (ADSC) culture supernatant, and whey protein-derived exosomes (WP-EXO). Among these, the company registered the patents for the manufacturing method of exosomes derived from NK cell culture supernatant and the cosmetic composition containing exosomes produced using this method. In addition, a patent application is underway for the recently established core technology of manufacturing exosomes derived from Wharton’s jelly mesenchymal stem cells (WJ-MSCs).
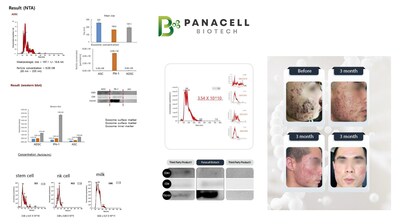
The core of Panacell Biotech’s exosome manufacturing patent is the separation of exosome particles up to 4×10¹¹ (400 billion)/mL using a filtration method from the culture medium obtained during the co-culture process of NK cells, NKT cells, and T cells extracted from a patient’s adipose tissue or blood.
The filtration device employs a method suitable for high-concentration samples by forming a fluid flow parallel to the filtration membrane to prevent fouling on the membrane surface. This method has been proven by extracting ultra-high-concentrated exosomes and analyzing them through the western blot.
Dr. Seung Ho Choi stated that, beyond treating domestic patients, the company has secured stem cell-related technology to a completion stage. With this technology, they recently attracted an investment of $5 million from the United States. They also signed memorandums of understanding to promote international technology transfers worth $12 million each to Malaysia and China.
Panacell Biotech also discovered multiple exosomes within the culture medium through component analysis. They have secured technologies for filtration, storage, mass production, and inspection for export and are currently operating a subsidiary established in New York.
Dr. Seung Ho Choi said, “Based on our experience researching and developing exosomes derived from human cells, we expect to see the fruition of developing therapeutics for rare and intractable diseases. We can achieve this by jointly working with domestic and international research institutions and medical organizations to create exosomes loaded with small interfering ribonucleic acid (si-RNA) that inhibits messenger RNA (mRNA) protein synthesis, utilizing exosomes as the core technology.”
Exosome cosmetics contain exosomes, nano-sized particles secreted by cells that include various bioactive substances such as proteins, nucleic acids, and lipids. These bioactive substances can exhibit multiple skin improvement effects, including skin regeneration, anti-inflammation, anti-oxidation, and whitening.
Since the effects of exosomes replicate the characteristics and functions of the original cells and vary significantly with concentration, the functionality of the cosmetics can also differ. Exosomes have a high skin absorption rate and can deliver bioactive substances to the deeper layers of the skin. With a structure similar to cells, they possess high skin compatibility and can solve the problem of low absorption rates of 1–3% caused by skin structure.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/the-exosome-production-method-patent-by-panacell-biotech-registered-as-original-technology-302266231.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/the-exosome-production-method-patent-by-panacell-biotech-registered-as-original-technology-302266231.html
SOURCE Panacell Biotech Co., Ltd.