FDA Clears Magstim Horizon INSPIRE Transcranial Magnetic Stimulation System to treat Depression, OCD, Anxious Depression

Next Generation Compact TMS System Offers Physicians, Nurse Practitioners, New Easy to Use, Affordable Effective Treatment
MINNEAPOLIS and CARMARTHENSHIRE, United Kingdom, Nov. 12, 2024 (GLOBE NEWSWIRE) — Magstim, the global leader in neuroscience research and treatment for mental health, has been awarded FDA clearance for the Horizon Inspire System, continuing to provide physicians, nurse practitioners, clinicians, and researchers with next-generation transcranial magnetic stimulation technology to treat patients with MDD, OCD and Anxious Depression*.
“Physicians, nurse practitioners and mental health care professionals tell us that patients are searching for alternatives to pharmaceutical treatments,” said Ronnie Stolec-Campo, CEO, Magstim. “FDA cleared transcranial magnetic stimulation is a proven and effective treatment with minimal side effects. We designed the Inspire to enable both experienced TMS providers as well as those who are new to TMS.”
According to Stolec-Campo, professionals requested a system to quickly launch their TMS practice. The Inspire addresses their needs with high-power air-cooling, ability to deliver back-to-back customizable treatments, all while being easy to use, cost-effective and offering portability between clinic rooms.
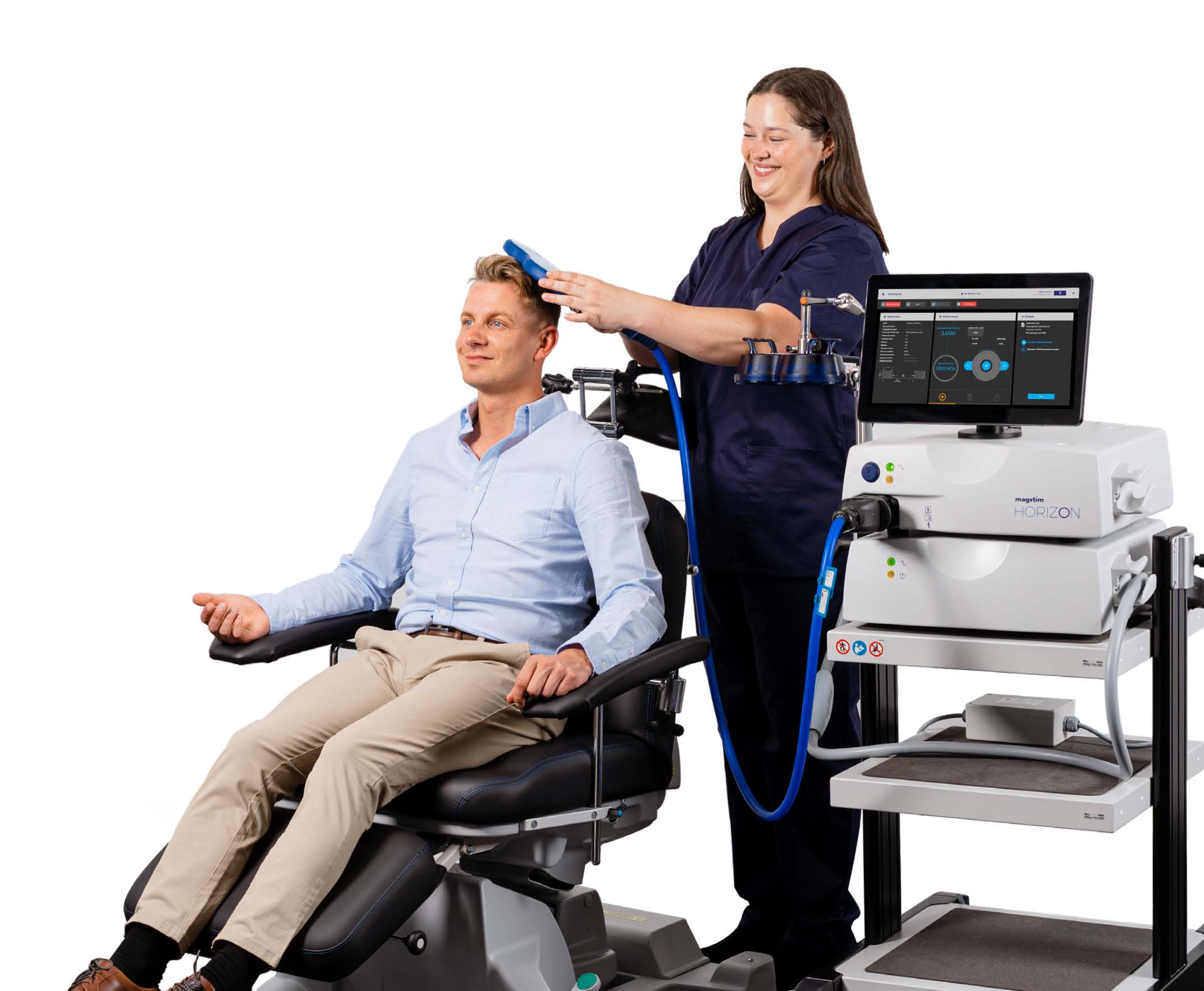
The Inspire is built on Magstim TMS technology, cited in more than 20,000 peer reviewed research papers and used in hospitals, clinics and research centers worldwide. The Inspire offers a powerful and versatile system to comfortably treat patients with mental health disorders.
The use of Transcranial Magnetic Stimulation for treating MDD and OCD is increasing worldwide, driven by new studies demonstrating its effectiveness over other treatments (**). TMS is now covered by most insurance companies, including Medicare. Many states now permit both physicians and psychiatric nurse practitioners (PMHNP) to prescribe and treat patients with TMS.
The Inspire system leverages intuitive preset clinical workflows to simplify the treatment process, delivering precise results with no pulse decay, ensuring the correct dosage. Magstim’s air-cooled coil reduces downtime and eliminates additional cooling expenses. Advanced data analytics tools improve treatment efficacy.
“Magstim engineered the very first commercially available TMS research technology, and we remain committed to our foundation of research” said Stolec-Campo. “We are unique in the industry because we do not charge pay per use fees, we maintain a dedicated service and support team, and we manufacture our own technology.”
Sign up for the November 20 Inspire education webinar at https://us06web.zoom.us/webinar/register/WN_PMoLw8jqRZajUt3fbjZLsQ
Visit Magstim.com or call 844-624-7846.
About Welcony
Globally, Welcony technologies support thousands of research labs, clinics, hospitals and universities that focus on mental health, brain disorders, cognitive neuroscience and neuromonitoring. Key brands include Magstim Magnetic Stimulation, MagstimEGI high-density EEG, Technomed Clinical Neurophysiology and Neurosign Neuromonitoring. Welcony is backed by Telegraph Hill Partners, a San Francisco based private equity company.
* Horizon 3.0 Inspire is indicated for the treatment of MDD in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode, as well as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD).
** https://www.psychiatrictimes.com/view/rtms-for-antidepressant-nonresponders
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/24ecc4b8-6d08-4d8c-a757-5cf290421aaa
CONTACT: Media Contact: Mark Sejvar, mark.sejvar@magstimegi.com, 323-363-3530

