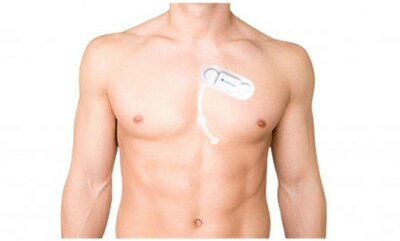
SmartCardia Receives FDA Clearance for Outpatient Cardiac Telemetry for Its 7-Lead ECG Patch and Cloud Platform

LAUSANNE, Switzerland, Nov. 13, 2024 /PRNewswire/ — SmartCardia has received FDA clearance for Mobile Outpatient Cardiac Telemetry (OCT/MCT) for its 7-lead live ECG monitoring patch and cloud platform. SmartCardia’s 7L patch is easy-to-wear, cable-free, waterproof and can be used for continuous monitoring for up to 14 days. This comes on the heels of SmartCardia’s prior FDA clearance for Extended Holter, Event and Holter monitoring. With this approval, SmartCardia’s solution can be used for remote live monitoring of patients’ ECG and immediately notify clinicians for important arrhythmias.
SmartCardia’s patch and cloud platform is a single solution for complete cardiology practice: the patch can be used in any of the modes from holter to mobile telemetry and can also be remotely transitioned between them seamlessly for patients. The 7 ECG leads are streamed live to the cloud, where the ECG is immediately available. The proprietary cloud platform analyses the ECG live to provide outpatient telemetry notifications and full disclosure ECG analysis. The 7L platform features real-time view of the patient’s ECG and can deliver visual and audio alarms.
“SmartCardia’s platform effectively addresses the primary needs of today’s electrophysiologists and cardiologists,” said Dr. Jag Singh from Harvard Medical School and Massachusetts General Hospital and Principal Advisor of SmartCardia. “Its 7-lead ECG delivers unparalleled clinical accuracy, the live monitoring allows for immediate notifications, and the reusable sensor with a low cost disposable patch promotes high operational efficiency.”
All ECG data—not just events—is transmitted live, with automated analysis applied continuously across the entire signal. This full-disclosure analysis enables highly accurate arrhythmia detection, and the cloud platform’s intuitive navigation allows clinicians to easily review and analyse ECG data at any moment.
“Many products available on the market do not have explicit FDA clearance for outpatient cardiac telemetry. Our patch and cloud platform are designed from the ground up to meet stringent outpatient telemetry standards, and this clearance validates our commitment and efforts. With an end-to-end patch to cloud, our solution provides accurate and on time detection of arrhythmia events”, said Adam Kirchgessner, SmartCardia’s US VP of Sales.
SmartCardia has recently completed acquisition of a US Independent Diagnostic Testing Facility (IDTF) closing the loop to become best-in-class provider with control over the full ecosystem. SmartCardia has “in-network” coverage with major insurances for the different monitoring modalities across US.
SmartCardia’s solution has CE Class IIa approval in Europe for ECG, analysis, cloud and vital signs. SmartCardia is expanding globally, with broad adoption across multiple countries and is rapidly entering new regions. The company was honoured with the 2022 Global New Product Innovation Award in cardiac monitoring from Frost & Sullivan.
About SmartCardia:
SmartCardia is a leading provider of an integrated cloud platform and patch for cardiac and remote patient monitoring. Integrating accurate ECG and vital sign data with clinician cloud SaaS platform enables SmartCardia to provide actionable insights into patients’ health. The solution provides excellent clinical, operational, and financial benefits to hospitals and patients globally.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/smartcardia-receives-fda-clearance-for-outpatient-cardiac-telemetry-for-its-7-lead-ecg-patch-and-cloud-platform-302303172.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/smartcardia-receives-fda-clearance-for-outpatient-cardiac-telemetry-for-its-7-lead-ecg-patch-and-cloud-platform-302303172.html
SOURCE SmartCardia