Theralase(R) Demonstrates Unique Ability to Activate Rutherrin(R) With Diabetes Drug

TORONTO, ON / ACCESSWIRE / August 21, 2024 / Theralase® Technologies Inc. (“Theralase®” or the “Company“) (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation-activated small molecules for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that it’s lead drug formulation, Rutherrin® has been proven preclinically to be activated by Metformin, a common diabetes drug, without the use of light and/or radiation.
Even more interesting is that because Rutherrin® and Metformin are both scientifically proven to cross the blood-brain barrier, as well as tumour-specific blood barriers, this new discovery potentially allows the precise targeting of cancer cells by Rutherrin® anywhere inside the body, including the brain, followed by their synergistic activation by Metformin.
Metformin, an anti-diabetic agent, was approved by the U.S. Food and Drug Administration in 1994 for the treatment of Type 2 diabetes (a medical condition resulting from the insufficient production of insulin, causing high blood sugar). Metformin is currently the only anti-diabetic medication prescribed for the prophylactic (intended to prevent disease) treatment of prediabetes, as recommended by the American Diabetes Association.
In order to determine if Metformin has the ability to activate Rutherrin® to produce Reactive Oxygen Species (“ROS“), for the destruction of treatment resistant, Non-Small Cell Lung Cancer (“NSCLC“) cells, NSCLC cells were treated with either Rutherrin®, Metformin or a combination of both for 24 hours.
As can be seen in Figure 1.0, Rutherrin® on its own increases ROS production, without any external stimuli, leading to cancer cell death; however, this ROS production is significantly increased by activating Rutherrin® with Metformin (p<0.001).
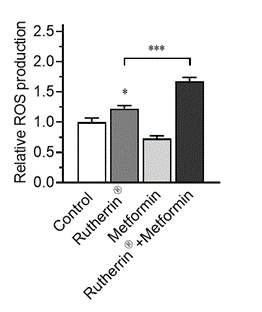
Figure 1.0: Reactive Oxygen Species Production in NSCLC Cells Treated with Rutherrin®, Metformin or Their Combination
To further explore the activation of Rutherrin® by Metformin, an additional set of experiments were conducted, where the cells were treated with either Rutherrin®, Metformin or both for 24 hours and then irradiated with radiation.
As can be seen in Figure 2.0, as expected, radiation-activated Rutherrin® increases ROS production (p<0.01); however, ROS production is significantly increased with radiation-activated Rutherrin® when combined with Metformin (p<0.001).
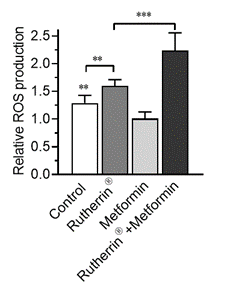
Figure 2.0: Reactive Oxygen Species Production in NSCLC Cells Treated with Radiation-Activated Rutherrin®, Metformin or Their Combination
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated, “The ability to activate Rutherrin®, with commonly available drugs like Metformin, significantly opens the clinical applications of Rutherrin®, in the destruction of various cancers. Rutherrin®, when activated by light and/or radiation, leads to the production of ROS, which is one of the key Mechanisms of Action in the destruction of cancer by the compound. Synergistic activation of Rutherrin®, using a drug, such as Metformin, with or without radiation exposure, opens up a wide range of advantageous opportunities for practitioners to treat patients outside the operating room. In addition, this latest research supports a whole new line of research; specifically, identifying additional drugs, as potential synergistic candidates for activating Rutherrin® in the destruction of various cancers.”
Roger DuMoulin-White, B.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated, “The activation of Rutherrin® by Metformin is a game changer in the clinical use of Rutherrin®. By activating it, without light and/or radiation, this treatment can significantly be expanded to offering it on an out-patient basis or even an in-home treatment basis. Traditionally, Rutherrin®, is required to be activated by laser light and/or radiation, but now it is envisioned that Rutherrin® can be instilled in the body via IV drip followed by activation with Metformin, taken orally. This can be accomplished virtually anywhere, and if required, can be accompanied by hospital-based radiation treatments, if the disease state requires it. Based on the latest research, pending Good Laboratory Practices toxicology analysis of Rutherrin® and regulatory approval, Theralase® plans to commence practitioner-led and/or Theralase®-led, clinical studies in the destruction of various cancers. The clear advantage is that light and/or radiation equipment is no longer a prerequisite and the procedure can be completed outside of the hospital or expensive operating room, significantly lowering the cost of providing the treatment and the burden on elderly patients, with limited mobility. We look forward to commercializing this technology and its ease of delivery for the benefit of all patients stricken with this terrible disease.”
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation and drug activated compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements
This news release contains Forward-Looking Statements (“FLS“) within the meaning of applicable Canadian securities laws. Such statements include, but are not limited to, statements regarding the Company’s proposed development plans with respect to small molecules and their drug formulations. FLS may be identified by the use of the words “may, “should“, “will“, “anticipates“, “believes“, “plans“, “expects“, “estimate“, “potential for” and similar expressions; including, statements related to the current expectations of the Company’s management for future research, development and commercialization of the Company’s small molecules and their drug formulations; including: preclinical research, clinical studies and development and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, whether the Company is able to: adequately fund and secure the requisite regulatory approvals to successfully complete preclinical and clinical studies in a timely fashion to implement its development plan; successfully commercialize its drug formulations; access sufficient capital to fund the Company’s operations, which may not be available on terms that are commercially favorable to the Company or at all; provide preclinical and clinical support that the Company’s drug formulations are effective against the conditions tested in its preclinical and clinical studies; comply with the term of license agreements with third parties, not to lose the right to use key intellectual property in its business; protect its intellectual property and the timing and success of this intellectual property and achieve acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company’s ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will successfully come to fruition, and as such, FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For More Information:
1.866.THE.LASE (843.5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
khachey@theralase.com
416.699.LASE (5273) x 224
SOURCE: Theralase Technologies, Inc.
View the original press release on accesswire.com
