Clinical Trial Management System (CTMS) Market size is set to grow by USD 1.86 billion from 2024-2028, Increasing healthcare expenditure boost the market, AI Role and Impact, Technavio

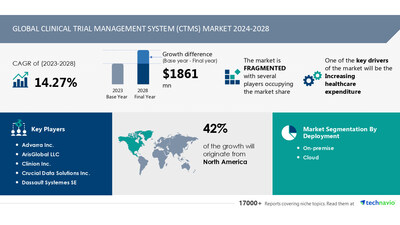
NEW YORK, Aug. 23, 2024 /PRNewswire/ — The global clinical trial management system (CTMS) market size is estimated to grow by USD 1861 mn from 2024-2028, according to Technavio. The market is estimated to grow at a CAGR of almost 14.27% during the forecast period. Increasing healthcare expenditure is driving market growth, with a trend towards increasing outsourcing of clinical trial process. However, rising cost of clinical trials poses a challenge. Key market players include Advarra Inc., Aris LLC, Clinion Inc., Crucial Data Solutions Inc., Dassault Systemes SE, DATATRAK International Inc., DSG Inc., Ennov SAS, eResearchTechnology GmbH, International Business Machines Corp., Laboratory Corp. Of America Holdings, MasterControl Solutions Inc., Medfiles USA, MedNet, Oracle Corp., Parexel International Corp., PHARMASEAL, RealTime Software Solutions LLC, Veeva Systems Inc., and Wipro Ltd..
Get a detailed analysis on regions, market segments, customer landscape, and companies- View the snapshot of this report
|
Clinical Trial Management System (CTMS) Market Scope |
|
|
Report Coverage |
Details |
|
Base year |
2023 |
|
Historic period |
2018 – 2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 14.27% |
|
Market growth 2024-2028 |
USD 1861 million |
|
Market structure |
Fragmented |
|
YoY growth 2022-2023 (%) |
12.65 |
|
Regional analysis |
North America, Europe, Asia, and Rest of World (ROW) |
|
Performing market contribution |
North America at 42% |
|
Key countries |
US, Germany, China, Canada, and UK |
|
Key companies profiled |
Advarra Inc., ArisGlobal LLC, Clinion Inc., Crucial Data Solutions Inc., Dassault Systemes SE, DATATRAK International Inc., DSG Inc., Ennov SAS, eResearchTechnology GmbH, International Business Machines Corp., Laboratory Corp. Of America Holdings, MasterControl Solutions Inc., Medfiles USA, MedNet, Oracle Corp., Parexel International Corp., PHARMASEAL, RealTime Software Solutions LLC, Veeva Systems Inc., and Wipro Ltd. |
Market Driver
In the pharmaceutical industry, the shift towards outsourcing drug discovery processes to subcontract laboratories has gained significant traction over the last decade. This trend has been driven by the need for increased R&D investments and the high cost of building and maintaining in-house research facilities. As a result, numerous small companies have opted to focus on manufacturing and marketing, while outsourcing testing and research to external partners. The number of subcontract laboratories has surged since 2011, and this trend is expected to continue. This outsourcing model has enabled companies to carry out research and testing at a fraction of the cost of building and maintaining their own facilities. The popularity of this approach is not limited to developed countries like the US, the UK, Germany, and Japan, but has also gained acceptance in emerging economies such as India and Brazil. The high volume of data generated during the drug discovery process necessitates the use of Clinical Trial Management Systems (CTMS) to manage and analyze data effectively. Consequently, the growing outsourcing trend is fueling demand for CTMS solutions in the pharmaceutical industry.
The Clinical Trial Management System (CTMS) market is experiencing significant trends that are shaping the future of clinical trials. Robust reporting and centralizing recruitment are key priorities for multinational companies and local CROs alike. ERegulatory, eSOURCE, accounting, and aggregate reporting are essential features for ensuring regulatory compliance and trial efficiency. Digitalization, personalized medicine, and decentralized trials are driving innovation, with outsourcing and externalization becoming more common. Cloud-based CTMS, telemedicine solutions, and decentralized clinical trials are on the rise, especially in the context of vaccines and COVID-19 trials. Investment in AI and advanced technologies is also increasing to streamline processes and reduce costs. System security, backups, upgrades, and uptime consistency are crucial for maintaining trust and confidence in CTM systems. Regulatory policies continue to evolve, requiring ongoing oversight and adaptation. Overall, the market for CTM systems is growing, with trends including ERT, Bioclinica, Healthcare IT, and network-wide visibility.
Explore a 360° Analysis of the Market: Unveil the Impact of AI. For complete insights- Request Sample!
Market Challenges
- Clinical trials are a crucial part of bringing new drugs to market, but their rising costs are a significant concern for pharmaceutical companies. One major factor contributing to these costs is the difficulty and lengthy process of patient enrolment and retention. According to various studies, the average cost per patient in clinical trials in the US is approximately USD41,117, with phase 3 trials being the most expensive, costing around USD20 million. Another factor is the increasing need for extensive clinical data collection due to new regulations, leading to complex and costly trials, particularly for chronic diseases. The longer the trial duration, the higher the costs, making some clinical research associates prefer manual work over management systems for cost-cutting. To mitigate these costs, it’s essential to increase patient awareness and encourage more participation. Pharmaceutical companies should focus on educating patients rather than persuading them, leading to a potential solution for the unhindered growth of the clinical research industry.
- In the dynamic healthcare IT landscape, Clinical Trial Management Systems (CTMS) have become essential tools for managing complex clinical trials. However, implementing and managing CTMS comes with challenges. Decentralized trials, vaccine development, and telemedicine solutions have increased the need for cloud-based CTMS. Costs, system security, backups, and upgrades are key concerns. Uptime consistency, centralization of data, growth, and automation are important for clinical trial managers. AI and machine learning algorithms streamline processes, but ensure access to data on devices like mobile workstations, laptops, tablets, and online dashboards. Trial planning, monitoring activities, regulatory procedures, supplies, finance, and subscription-based software upgrades require careful consideration. Scalability, add-ons, regulatory norms, document management processes, data quality, participant safety, and adherence to regulatory norms are ongoing challenges. Solutions like Clinion’s CTMS, IWRS/RTSM, EDC, eCOA, and accruals/deviations systems help address these challenges.
For more insights on driver and challenges – Request a sample report!
Segment Overview
This clinical trial management system (ctms) market report extensively covers market segmentation by
- Deployment
- 1.1 On-premise
- 1.2 Cloud
- 2.1 Pharmaceutical and biotechnology companies
- 2.2 CROs
- 2.3 Others
- 3.1 North America
- 3.2 Europe
- 3.3 Asia
- 3.4 Rest of World (ROW)
1.1 On-premise- The Clinical Trial Management System (CTMS) market primarily consists of on-premises and cloud-based deployment models. While on-premises CTMS offers enhanced data security and control, it necessitates substantial investments in infrastructure and maintenance costs. These factors may impede the growth of the CTMS market during the forecast period. However, large firms with adequate resources prefer on-premises solutions due to the perceived higher level of data security. Notable on-premises CTMS vendors include Siebel and IMPACT. Despite the challenges, the on-premises segment is expected to expand in the global CTMS market due to the security benefits it provides to large organizations.
For more information on market segmentation with geographical analysis including forecast (2024-2028) and historic data (2017-2021) – Download a Sample Report
Research Analysis
The Clinical Trial Management System (CTMS) market is a significant segment of Healthcare IT, enabling the efficient and effective management of clinical trials. With the increasing focus on decentralized clinical trials and the development of vaccines, the demand for advanced CTMS solutions is surging. Cloud-based CTMS is gaining popularity due to its accessibility and scalability, while telemedicine solutions facilitate remote patient monitoring. IWRS/RTSM, EDC, eCOA, accruals, deviations, and regulatory compliance are essential features of CTMS. Robust reporting, outsourcing, and externalization are key considerations for multinational companies and local CROs. The market is witnessing significant investment, driven by the need for scalability and regulatory policies. Clario and other innovative solutions are transforming CTMS with features like real-time data access and integration with ERT.
Market Research Overview
The Clinical Trial Management System (CTMS) market is a significant segment of Healthcare IT, witnessing robust growth due to the increasing adoption of decentralized clinical trials, particularly in the development of vaccines. CTMS solutions are increasingly moving to cloud-based platforms, enabling telemedicine solutions and remote monitoring. The COVID-19 pandemic has accelerated this trend, with the need for remote trial management and data access. CTM systems facilitate trial planning, monitoring activities, regulatory procedures, supplies management, finance, and accounting. They offer centralization of data, ensuring uptime consistency, system security, backups, and upgrades. AI, machine learning algorithms, automation, and digitalization are key features, enabling clinical trial managers to access data from devices, mobile workstations, laptops, tablets, and online dashboards. Costs, system security, and data quality are critical factors, with subscription-based models, software upgrades, add-ons, and regulatory norms influencing market growth. CTMS systems include IWRS/RTSM, EDC, eCOA, accruals, deviations, scalability, and robust reporting. Centralizing recruitment, eRegulatory, eSOURCE, accounting, aggregate reporting, network-wide visibility, and oversight are additional benefits. The market caters to multinational companies, local CROs, and outsourcing entities, with decentralized trial models, evidence-based facilities, and regulatory policies driving investment.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- Deployment
- On-premise
- Cloud
- End-user
- Pharmaceutical And Biotechnology Companies
- CROs
- Others
- Geography
- North America
- Europe
- Asia
- Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/clinical-trial-management-system-ctms-market-size-is-set-to-grow-by-usd-1-86-billion-from-2024-2028–increasing-healthcare-expenditure-boost-the-market-ai-role-and-impact-technavio-302228536.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/clinical-trial-management-system-ctms-market-size-is-set-to-grow-by-usd-1-86-billion-from-2024-2028–increasing-healthcare-expenditure-boost-the-market-ai-role-and-impact-technavio-302228536.html
SOURCE Technavio