Bruder Healthcare Introduces Eyeleve MGD, a Trusted Eye Drop Formula Under a New Brand

With 80 percent of dry eye patients suffering from evaporative dry eye, Eyeleve MGD will serve a crucial need of patients and practitioners alike.
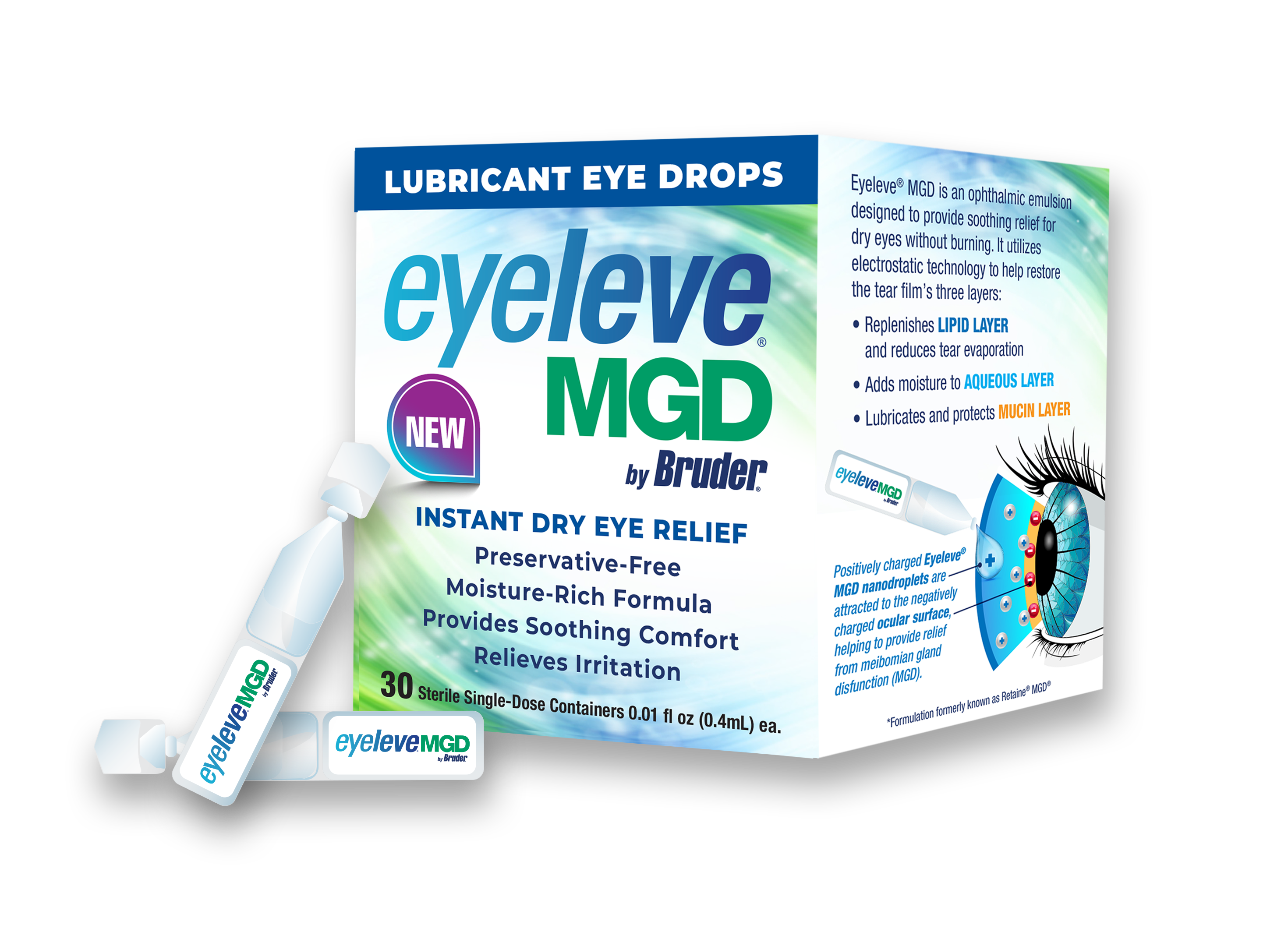
ATLANTA, GA / ACCESSWIRE / August 27, 2024 / Bruder Healthcare, a Hilco Vision Company, is pleased to announce that it has entered into an exclusive distribution agreement with Santen S.A. to market Eyeleve® MGD in the United States. To be marketed in early 2025 as Eyeleve® MGD, the eye drop formulation was previously marketed in the United States as Retaine® MGD. This innovative preservative-free ophthalmic emulsion provides moisturization, lubrication, and protection for moderate to severe evaporative dry eye (EDE). With 80 percent of dry eye patients suffering from EDE, Eyeleve MGD will serve a crucial need of patients and practitioners alike.
Eyeleve MGD by Bruder, preservative-free eye drops for moderate to severe evaporative dry eye
How it works
The formulation uses a preservative-free oil-in-water cationic emulsion technology. This technology delivers ingredients through electrostatic attraction between positively charged droplets and the negatively charged ocular surface. This innovative approach promotes relief from dry eye symptoms by moisturizing, lubricating, and protecting the eyes.
Hilco Vision’s Brent Jones, Global Head of Vision Care Solutions, commented, “We are thrilled to introduce Eyeleve MGD to our portfolio of dry eye treatments. Adding Eyeleve MGD strengthens our product line and better equips eye care professionals to provide solutions for patients on the road back to healthy eyes.”
Research-backed
The hypotonicity of the emulsion in Eyeleve MGD adds moisture by lowering the salt concentration of tears, while the lipid component lubricates and protects the surface of the eye. This unique combination of ingredients ensures that patients experience relief from dry eye symptoms, improving their overall eye health and comfort.
As co-author of a study published in Clinical Ophthalmology, Dr. Paul Karpecki states that study data demonstrated a therapeutic effect of the formulation on the signs and symptoms of dry eye after two weeks of treatment. In addition, study participants experienced an immediate-onset transient improvement in tear film stability. “This preservative-free drop not only addresses the physiological challenges of dry eye but also enhances patients’ quality of life, particularly in activities like prolonged computer use,” adds Karpecki, underscoring its role as a promising option in the battle against dry eye syndrome1.
Bruder Healthcare and Hilco Vision are committed to delivering top-tier dry eye treatments to patients and practitioners. The upcoming launch of Eyeleve MGD in early 2025 reinforces this dedication. With innovative solutions like Eyeleve MGD, Hilco Vision continues to enhance its market-leading, comprehensive approach to dry eye care.
For more about Eyeleve MGD, visit www.eyeleve.com. For more information about Bruder Healthcare, a Hilco Vision Company, and their dry eye treatment offerings, visit www.bruderpro.com or www.hilcovision.com.
1 Clin Ophthalmol. 2015; 9: 235-243.
Contact Information
Stan Samples
Senior Mgr, Marketing Communications
stan.samples@bruder.com
770-410-0726 x231
Related Files
Brent-Jones (2)
Paul-Karpecki-2024
SOURCE: Bruder Healthcare
View the original press release on newswire.com.
