As Research into Pain Accelerates, a Newly Recognized Type of Pain Comes to the Forefront – Tonix Targets it with FDA-Fast Track-Designated TNX-102 SL

CHATHAM, NJ / ACCESSWIRE / September 4, 2024 /

This post was written and published as a collaboration between the in-house editorial team at Benzinga and Tonix Pharmaceuticals Holding Corp. with financial support from Tonix. The two organizations work to ensure that any and all information contained within is true and accurate as of the date hereof to the best of their knowledge and research. This content is for informational purposes only and not intended to be investing advice.
Research into the causes and nature of pain is accelerating and broadening as evidenced by the more than 400 scientific presentations at the 2024 World Congress on Pain, held August 6-9 in Amsterdam, which was sponsored by the International Association for the Study of Pain.
The keynote plenary session lecture at the event put a spotlight on what researchers have named nociplastic pain, a third type of pain that is different than the two more well known types of pain, nociceptive (arising from a burn or cut), and neuropathic (arising from pinched or inflamed nerves).
The hallmark of nociplastic (no-see-plastic) pain is the experience of widespread pain in the absence of actual or threatened tissue damage. Nociplastic pain results from dysfunctional processing of sensory experiences by the nervous system. In mechanistic terms, it is a painful experience caused by the altered functioning of pain-related pathways in the peripheral and central nervous system.
Nociplastic pain can occur in isolation, as is the case with fibromyalgia, but an even greater number of people have mixed pain states. It’s common for people to become afflicted with nociplastic pain as parts of their body’s sensory system break down from years of suffering chronic nociceptive or neuropathic pain1.
“Nociplastic pain is the third primary type of pain and one of the most challenging to treat. Nociplastic pain is always chronic, so treatments need to be suitable for long term use. Tolerability and low-addiction risk are very important. Typical analgesics like NSAIDs and opioids miss the mark because of issues with long term tolerability and addiction, respectively,” notes Seth Lederman, M.D., CEO of Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP), which is developing a new non-addictive treatment for fibromyalgia, the prototypic nociplastic pain syndrome.
He continued, “Think of nociplastic pain as being caused by a software bug in a certain part of the brain that exaggerates sensory experiences and turns them into feelings of real pain.”
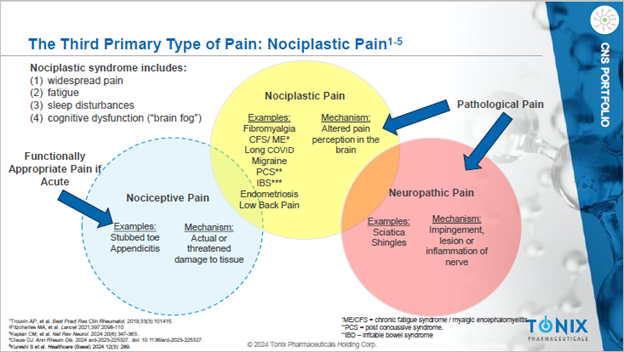
Fibromyalgia was the “Rosetta Stone” that led experts to decipher nociplastic pain and name it. People with fibromyalgia typically hurt all over their bodies, which is consistent with the programming flaw in the part of the brain that interprets all pain. Pain is like an error message, and coming from the brain the error message gets applied to almost all sensations.
The Three Kinds of Pain
-
Nociceptive pain is typified by a stubbed toe or appendicitis in which the nervous system is operating normally to warn about tissue injury and prevent further damage. It localizes the injury.
-
Neuropathic pain is typified by sciatica or shingles in which nerves are squeezed or infected by a virus. The pain experience can be localized to the injured tissue. But damaged nerves can also transmit location information that may be misleading – for example, in sciatica, pain shoots down the leg, while the problem is in compression on a spinal nerve root. Phantom Limb syndrome is another kind of neuropathic pain that appears to come from a limb that’s been amputated.
-
Nociplastic pain is the third type of pain, and the most recently defined. Until recently, people with nociplastic pain were treated with skepticism and disbelief because doctors, employers and insurance companies thought they were fabricating symptoms to avoid work, shirk responsibilities and seek benefits. However, nociplastic pain is very real to the patients who experience it2. Moreover, nociplastic pain may be even worse than nociceptive or neuropathic pain in terms of how many people suffer and for how long. More than 7 million people with nociplastic pain globally develop depression and anxiety. It is recognized as a major component of the opioid crisis.
Fibromyalgia is a common chronic pain condition that is the archetype of nociplastic pain.3,4,5 Three drugs are currently approved by the FDA, but patients and providers report widespread dissatisfaction with them due mainly to poor tolerability and off-putting side effects. As a result, doctors prescribe opioids off label more often than all of the FDA-approved drugs combined.
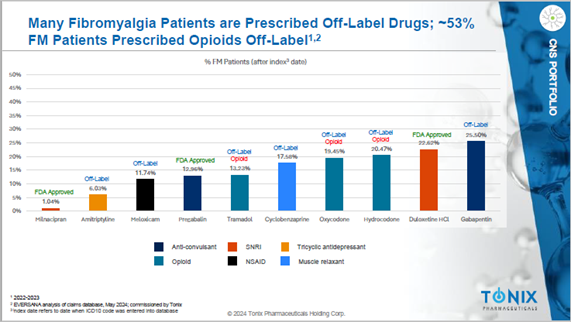
Opioids bring their own, well-known set of problems. They can be addictive and, over time, even life-threatening. Of patients prescribed opioids for chronic pain, 21% to 29% misuse them, while about 8% to 12% develop opioid use disorder. The cost of opioid addiction was close to $1.5 trillion in 2020 in the United States alone.
Tonix is developing TNX-102 SL*, a first-in-class non-opioid treatment for fibromyalgia. Since fibromyalgia is a chronic pain condition, TNX-102 SL is a potential non-opioid analgesic, which is the medical term for “pain killer”.
TNX-102 SL is Designed to Treat Fibromyalgia in a New Way
TNX-102 SL is a sublingual formulation of cyclobenzaprine hydrochloride designed to improve sleep quality rather than quantity in fibromyalgia, setting it apart from existing treatments for either fibromyalgia or sleep disorders.
Traditional sedatives like Ambien fail to manage the type of sleep disturbance that typifies fibromyalgia. Bad sleep worsens pain and pain worsens sleep6,7. Lack of sleep can also hyperexcite the central nervous system (CNS) lower pain tolerance. TNX-102 SL is designed to improve sleep quality in fibromyalgia and in two Phase 3 studies, TNX-102 SL improved nociplastic pain in fibromyalgia, which is the primary endpoint FDA requires to approve a new fibromyalgia drug.
In the latest phase 3 trial, TNX-102 SL showed a statistically significant improvement in fibromyalgia pain with a p-value of 0.00005. Tonix reports that significant results were also seen in improving sleep quality, reducing fatigue and improving overall fibromyalgia symptoms and function. TNX-102 SL was well tolerated and the most common side effects were transient sensations in the mouth corresponding with the disintegration of the tablet under the tongue.
An Urgent Need
The need for new ways to treat fibromyalgia is underscored by the fact that TNX-102 SL was recently granted Fast Track designation by the FDA, which is designed to facilitate development and expedite the review of important new drugs intended to treat serious conditions and address unmet medical needs. Its goal is to get new treatments to patients sooner. Companies whose programs are granted Fast Track designation are eligible for more frequent interactions with the FDA during clinical development.
In addition to receiving Fast Track designation, Tonix is finalizing the filing of the New Drug Application (NDA) for TNX-102 SL, which it plans to submit to the FDA in the second half of 2024. Coming out of pre-NDA meetings, Tonix said it is aligned with the FDA regarding TNX-102 SL. Tonix plans to request Priority Review designation for TNX-102 SL, and if granted, the FDA may accelerate the review of the NDA.
“The Fast Track designation underscores the importance of addressing the unmet needs of fibromyalgia patients, who report dissatisfaction with current treatment options,” said Dr. Lederman. “If approved by the FDA, we expect TNX-102 SL to become the first new pharmacotherapy for fibromyalgia in over 15 years.”
Nociplastic pain, whether it arises in a pure form from fibromyalgia or in a mixed form from another pathology, is a huge problem in America and beyond, negatively impacting the quality of life for millions of people. Current treatments are often either ineffective or difficult to take over long periods of time because of side-effects. TNX-102 SL, has no recognized risk of addiction and appears to be well tolerated with long term use. Tonix is preparing the NDA which has received FDA Fast Track designation. A new approach to fibromyalgia may mean hope for the millions of Americans who suffer from it.
*TNX-102 SL is an investigational new drug and has not been approved for any indication.
1Wikipedia “Nociplastic pain”. https://en.wikipedia.org/wiki/Nociplastic_pain.
2Geddes, L. June 28, 2021 “Sufferers of chronic pain have been told it’s all in your head. We now know that’s wrong. The Guardian. URL: www.theguardian.com/australia-news/2021/jun/28/sufferers-of-chronic-pain-have-long-been-told-its-all-in-their-head-we-now-know-thats-wrong
3Fitzcharles MA, et al. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 2021;397:2098-110
4Clauw DJ. From fibrositis to fibromyalgia to nociplastic pain: how rheumatology helped get us here and where do we go from here? Ann Rheum Dis. Published Online First: 2024
5Kaplan, C.M., Kelleher, E., Irani, A. et al. Deciphering nociplastic pain: clinical features, risk factors and potential mechanisms. Nat Rev Neurol. 2024;20, 347-363
6Moldofsky H, et al. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341-51
7Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35-44
Featured photo by monsitj on iStock.
This post contains sponsored content. This content is for informational purposes only and not intended to be investing advice.
Click here for more information on Tonix Pharmaceuticals:
https://redingtonvirtual.com/tnxp-aw-2409
Investor Contact
Jessica Morris
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
SOURCE: Tonix Pharmaceuticals Holding
View the original press release on accesswire.com
