Theralase(R) Provides Update on Bladder Cancer Clinical Study

TORONTO, ON / ACCESSWIRE / October 7, 2024 / Theralase® Technologies Inc. (“Theralase®” or the “Company“) (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that it is providing an update on its bladder cancer clinical study.
Theralase®’s lead drug, RuvidarTM, activated by the TLC-3200 Medical Laser System (“TLC-3200“) is currently under investigation in Canada and the United States in a Phase II registration study for Bacillus Calmette-Guérin (“BCG“)-Unresponsive Non-Muscle Invasive Bladder Cancer (“NMIBC“) Carcinoma In-Situ (“CIS“) with or without resected Ta / T1 papillary disease (“Study II“).
In the United States, an estimated 83,190 patients will be diagnosed with bladder cancer.1 Bladder cancer was the fourth leading cancer in men in 2023, representing 6% of estimated new cancers and 4% of cancer related deaths. At initial presentation, 70 to 75% of patients have NMIBC, 20 to 25% have Muscle Invasive Bladder Cancer (“MIBC“) and 5% have metastatic disease. CIS represents about 10% of NMIBC. CIS can progress at a five-year rate of 50%. Importantly, untreated patients with CIS can progress to MIBC at a 40 to 80% rate within five years.2
There is a critical need for effective bladder-sparing therapies for BCG-Unresponsive NMIBC.3BCG-Unresponsive NMIBC CIS is and remains a serious and life-threatening disease, which is an unmet need with a high treatment failure rate. The FDA has recognized the unmet medical need in the treatment of BCG-Unresponsive NMIBC with the issuance of their BCG-Unresponsive Guidance for Industry in August 2024.4
Study II is a Phase 2, single arm, open label clinical study for patients diagnosed with BCG-Unresponsive NMIBC CIS designed in compliance with the FDA’s guidance. The Study Procedure is comprised of the intravesical installation of reconstituted RuvidarTM for approximately 60 minutes activated by the intravesical TLC-3200 for approximately 60 minutes. 75 patients have currently been enrolled and have been provided the primary Study Procedure. 10 clinical study sites are currently enrolling patients in North America (5 in Canada, 5 in US). 100% of patients were provided the primary Study Procedure (RuvidarTM + TLC-3200) on Day 0 and 53.1% provided a re-induction Study Procedure. Patients are assessed according to standard of care at 90, 180, 270, 360 and 450 days. In response to a request by the FDA, the Company has tracked performance of these patients beyond 450 days; specifically: 540, 630, 720, 900 and 1080 days.
Primary Objective: Initial Efficacy(CR achieved at any point in time) determined by 1) Negative cystoscopy and negative cytology or 2) Positive cystoscopy (low grade disease) and negative cytology or 3) Negative cystoscopy and positive cytology (if random bladder biopsies are negative).
Secondary Objective: Duration of Efficacy(12 months duration of CR after diagnosis of initial CR) (15 months from primary Study Procedure).
Tertiary Objective: Safety (Incidence and severity of Adverse Events (“AEs“) > Grade 3, directly related to RuvidarTM or the TLC-3200, that do not resolve within 450 days post primary study treatment, where: Grade 1 = Mild, Grade 2 = Moderate, Grade 3 = Severe, Grade 4 = Life-threatening and Grade 5 = Death.
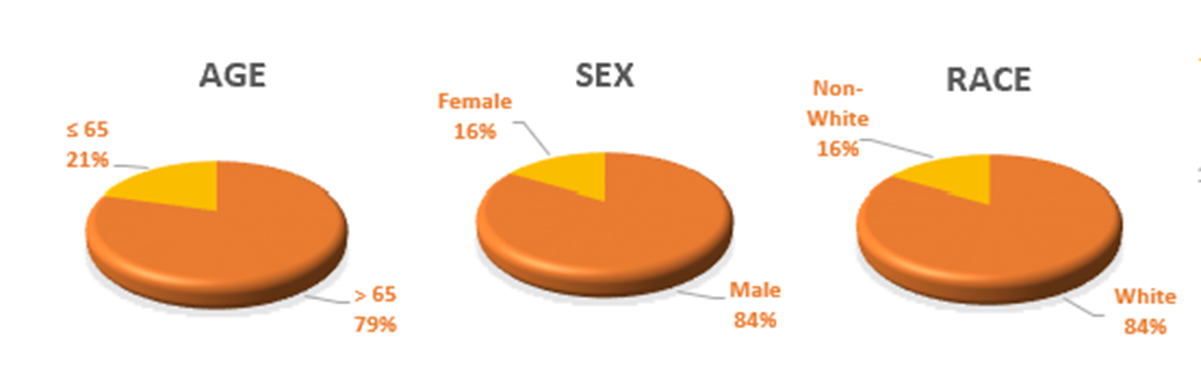
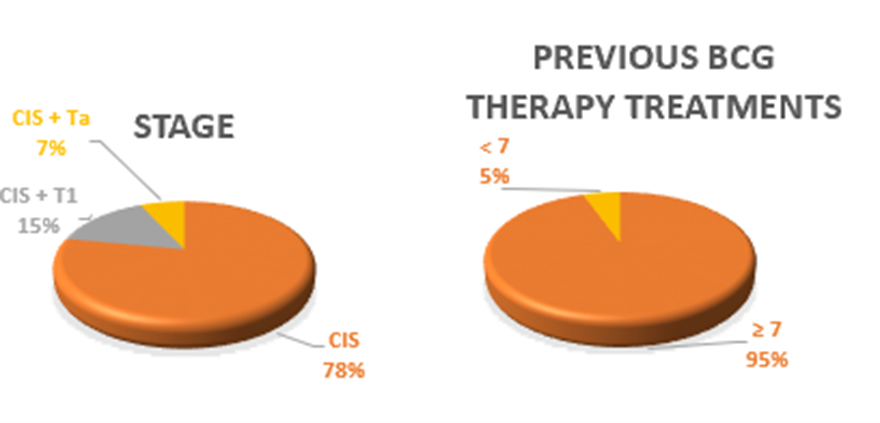
For patient demographics, 79% of patients enrolled are ≥ 65 years of age, 84% are male and 84% are white. All have been diagnosed with CIS +/- resected Ta/T1 disease. 95% are considered BCG-Unresponsive and 5% are considered BCG Intolerant. There are 75 patients (68 patients that have been evaluated, at least at the 90-day assessment).
The results of this interim analysis for the primary objective demonstrated a 60.3% (41/68) Complete Response (“CR“) [95% CI: 41.8%, 78.8%] at any point in time, comprised of patients achieving a CR at 90 days (37/68), at 180 days (3/68) and at 270 days (1/68).
For the secondary objective a CR of 26.5% (18/68) [95% CI: 14.2%, 38.7%] at 450 days was achieved.
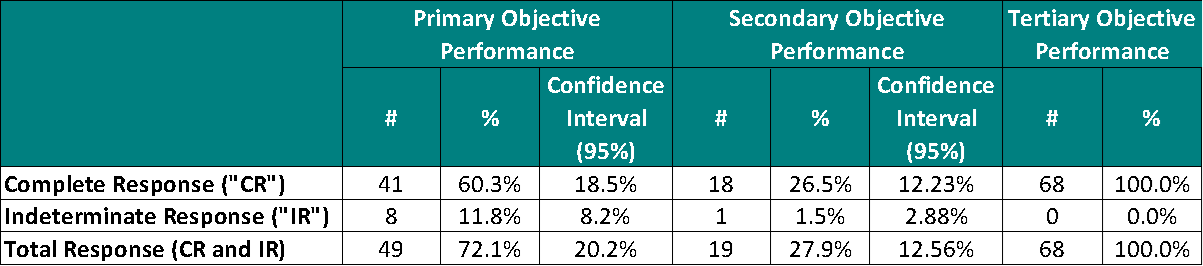
Note: Indeterminate Response (“IR“) is defined as negative cystoscopy (no evidence of Urothelial Cell Carcinoma (“UCC“) in the bladder) and positive urine cytology, suggesting UCC in the renal system other than the bladder).
In response to the FDA request, there is a demonstrated duration of CR of 13.2% (9/68) at 540 days, 8.8% (6/68) at 630 days, 7.4% (5/68) at 720 days, 7.4% (5/68) at 900 days and 5.9% (4/68) at 1080 days, with significant clinical data still pending.
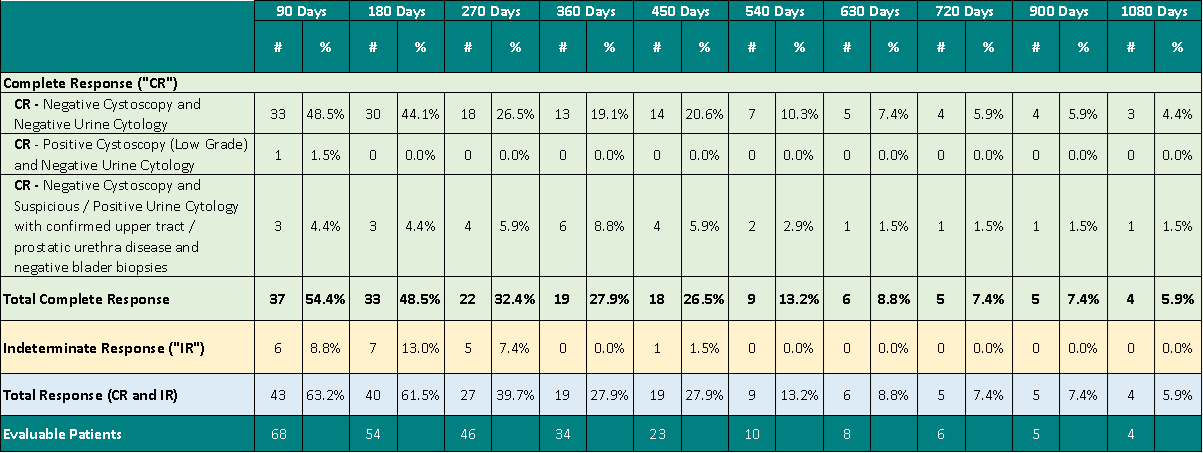
Analyzing Total Response (CR + IR) 72.1% (49/68) [95% CI: 51.9%, 92.3%] of patients achieved complete destruction of their bladder cancer at any point in time and 27.9% (19/68) [95% CI: 15.3%, 40.5%] had a duration of this response for at least 450 days, with significant clinical data still pending.
For the tertiary objective, there were 15 Serious Adverse Events (“SAEs“) involving 14 patients:
-
1 – Grade 1 (resolved in 9 days)
-
3 – Grade 2 (resolved within 1, 1 and 33 days, respectively)
-
7 – Grade 3 (resolved within 1, 2, 3, 4, 4, 82 and unknown days, respectively)
-
3 – Grade 4 (resolved within 3, 6 and 8 days, respectively)
-
1 – Grade 5
All SAEs were deemed unrelated / unlikely as a result of the Study Drug or Study Device.
In 2016, the International Bladder Cancer Group provided guidance that in this patient population (BCG-Unresponsive NMIBC CIS), an initial CR rate of 50% (Theralase® achieved 60.3%), with a 30% CR rate at 12 months (Theralase® achieved 27.9%) and 25% CR rate at 18 months (Theralase® achieved 26.5% at 450 days) would be desirable for an intervention to be widely adopted by the clinical profession.5
According to the interim clinical data, the Median Duration of Response achieved thus far is 13.1 months +/- 3.0.
All pathological clinical data received from the clinical study sites has been verified by central pathology.
Note: The clinical study is ongoing; thus, the clinical data presented is interim and represents clinical data collected to date. Evaluable patients represent patients assessed by a principal investigator.
In conclusion, the primary and secondary objectives presented in the interim clinical dataset have achieved internationally recommended guidelines; therefore, the primary and secondary objectives of the clinical study have been achieved.
The Theralase® Study Procedure (RuvidarTM and TLC-3200) is safe and effective in the treatment of BCG-Unresponsive NMIBC CIS and the clinical evidence indicates that the Study Procedure demonstrates an ability for patients to achieve CR and sustain a duration of that CR that is superior / comparable to FDA currently approved therapies, with significantly less Study Procedures.
A Duration of Response of ≥ 3 years is achievable after only one Study Procedure.
In response to this latest clinical data, Theralase® has submitted a pre-Break Through Designation (“BTD“) submission to the FDA to help identify and address any concerns of the FDA, prior to a formal BTD submission.
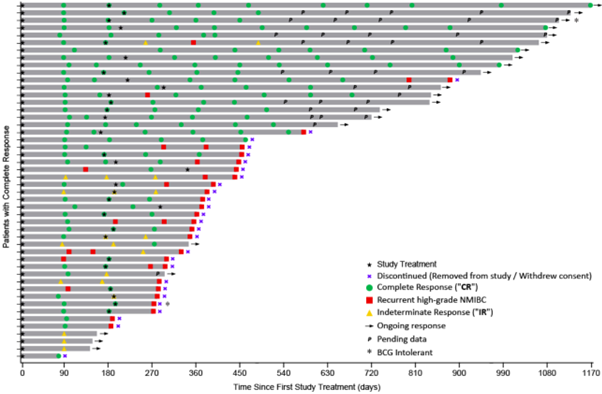
The Swimmer’s Plot below graphically displays the assessment of each patient who achieved a CR or IR response.
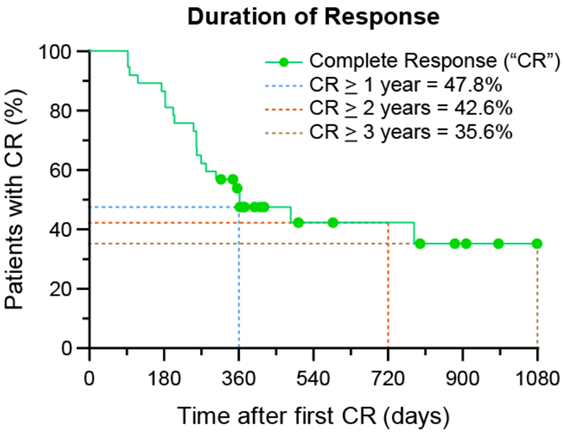
According to Kaplan-Meier’s Curve (which estimates the ability of patients to maintain their CR), the Duration of Response is ≥ 47.8% at 12 months, ≥ 42.6% at 24 months and ≥ 35.6% at 36 months.
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated, “I am pleased by the latest clinical data supporting the application of light-activated Ruvidar™ in the destruction of bladder cancer. BCG-Unresponsive NMIBC CIS is an extremely difficult subset of bladder cancer to treat, with five-year progression rate of 50% and if left untreated, an ability to progress to MIBC at a 40 to 80% rate within five years. The Theralase® technology has been proven to provide an opportunity for these patients to maintain their quality of life, through retention of their bladders for ≥ 3 years, after a single Study Procedure.”
Roger DuMoulin-White, B.E.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated, “This latest update on our bladder cancer clinical study continues to strengthen our understanding of the potency of Ruvidar™ in the destruction of bladder cancer. It is a very effective drug and when activated by laser light, even more effective, providing both high efficacy and a high safety profile for patients. As Theralase® completes enrollment in its clinical study, this year and into the beginning of next year, Theralase® is actively seeking partnering / licensing opportunities for various geographical territories around the world in the commercialization of Ruvidar™ for the treatment of BCG-Unresponsive NMIBC.“
1Key Statistics for Bladder Cancer | American Cancer Society (2024)
2 Lopez-Beltran A, Cookson M S, Guercio B J, Cheng L. Advances in diagnosis and treatment of bladder cancer BMJ 2024: 384 :e076743
3 Li, R., et al., Systematic Review of the Therapeutic Efficacy of Bladder-preserving Treatments for Non-muscle-invasive Bladder Cancer Following Intravesical Bacillus Calmette-Guérin. Eur Urol, 2020. 78(3): p. 387-399.
5 Kamat AM et al. J Clin Oncol. 2016; 34: 1935-1944
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedarplus.ca
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains “forward-looking statements” within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company’s proposed development plans with respect to small molecules and their drug formulations. Forward looking statements may be identified by the use of the words “may, “should“, “will“, “anticipates“, “believes“, “plans“, “expects“, “estimate“, “potential for” and similar expressions; including, statements related to the current expectations of Company’s management for future research, development and commercialization of the Company’s small molecules and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations, the risk that access to sufficient capital to fund the Company’s operations may not be available on terms that are commercially favorable to the Company or at all, the risk that the Company’s small molecule and drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company’s fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company’s ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company’s ability to control or predict.
Readers should not unduly rely on these forward-looking statements, which are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate as such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For investor information on the Company, please feel to reach out Investor Inquiries – Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
X 224
khachey@theralase.com
SOURCE: Theralase Technologies, Inc.
View the original press release on accesswire.com
