XPhyto Provides Update on Covid-19 Rapid Test Commercialization
VANCOUVER, BC / ACCESSWIRE / August 10, 2020 / XPhyto Therapeutics Corp. (CSE:XPHY / OTC PINK:XPHYF / FSE:4XT) (“XPhyto” or the “Company“), a next generation bioscience company, is pleased to announce an update on its rapid COVID-19 (SARS-COV-2) screening test, near-term milestones and the pathway to commercialization.
XPhyto and its exclusive diagnostic partner, 3a-Diagnostics GmbH (“3a”), are developing a rapid, disposable, point-of-care lateral flow screening test to detect COVID-19 viral RNA from patient saliva and nasal and throat swabs (the “Test”). On July 6, 2020, the Company announced successful validation of its working prototype for concurrent and independent detection of both the COVID-19 virus and viruses in the broader coronavirus family. 3a’s enhanced RNA probe system has demonstrated a detection limit capable of identifying viral RNA at concentrations found in the saliva of symptomatic, pre-symptomatic, and asymptomatic patients as observed and reported by clinicians and scientists in peer reviewed publications.[1][2][3]

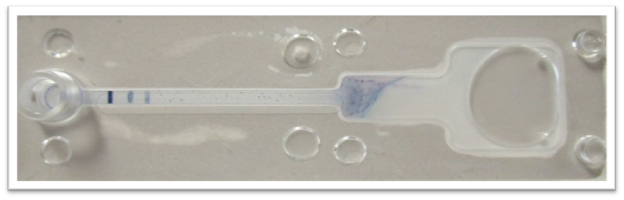
Figure 1. A photograph of the Test prototype with a positive signal for both COVID-19 RNA (genome sequence specific to SARS-COV-2) and universal coronavirus RNA (genome sequence shared across the coronavirus family). From left to right: Signal 1: COVID-19 probe, Signal 2: universal coronavirus probe, and Signal 3: control. The above results were visually confirmed in less than 5 minutes.
Test development and optimization is proceeding on an expedited basis at 3a’s research lab in Germany and in collaboration with third party contractors and academic partners. Subject to fast track certification by the German government, XPhyto and 3a are targeting Q1 2021 for European regulatory approval and commercial sales pursuant to the following estimated work schedule:
|
Development and commercialization milestones |
Start Date |
Finish Date |
Complete |
|
Product design and optimization |
04/20 |
11/20 |
|
|
Preliminary prototype production |
05/20 |
05/20 |
Yes |
|
Proof of concept (3a enhanced probes/synthetic RNA) |
06/20 |
07/20 |
Yes |
|
Proof of concept (reference RNA) |
07/20 |
08/20 |
|
|
Advanced prototype production (for usability testing) |
10/20 |
11/20 |
|
|
On site usability testing |
11/20 |
11/20 |
|
|
Product design freeze |
11/20 |
11/20 |
|
|
ISO 13485 audit (ISO certification) |
12/20 |
01/21 |
|
|
CE-IVD certification (CE mark commercial approval) |
01/21 |
02/21 |
|
|
Commercial sales |
02/21 |
– |
Three potential risk factors have been identified that could negatively impact the estimated schedule of milestones: 1) general internal and/or third party delays; 2) delays related to adjustments for usability optimization; and 3) delays associated with the ISO 13485 audit. XPhyto and 3a are actively working to mitigate delay risks and accelerate the estimated schedule wherever possible. The Company will provide ongoing milestone updates, including regarding clinical evaluation, in due course and as appropriate.
XPhyto and 3a are developing rapid screening tests for COVID-19 and other pandemic threats, including H1N1 (swine flu) and H5N1 (avian flu), with a specific focus on early pre-symptomatic stages of infection. Screening tests include later flow assay type tests as well as next-generation biosensors delivered via XPhyto’s dissolvable oral drug delivery platform. The product pipeline is comprised exclusively of rapid, low-cost, easy-to-use, saliva-based screening tools that can be self-administered, making them ideal for decentralized population scale screening.
Please join us today, Monday, August 10, 2020, for a live presentation at 12:30 PM EST: https://www.wallstreetreporter.com/next-superstock-online-investor-conference/.
The Company is not making any express or implied claims that its product has the ability to eliminate, cure or contain the COVID-19 pandemic.
About XPhyto Therapeutics Corp.
XPhyto is a diversified bioscience company with strategic assets and investments in the field of next generation drug delivery and rapid pathogen screening systems, as well as medical cannabis opportunities focused on European markets. Through its 100% owned subsidiaries and exclusive collaboration agreements, XPhyto is pursuing clinical programs for the transdermal and dissolvable oral delivery of conventional and cannabis based narcotics for neurological applications, as well as rapid dissolvable oral biosensor and lateral flow assay-based screening tests for dental health applications and high-risk pandemic threats such as SARS-COV-2 (COVID-19), H1N1 (swine flu) and H5N1 (avian flu). XPhyto has two exclusive cannabis collaborations with the Technical University of Munich, and two exclusive 5-year engagements with the University of Alberta, Faculty of Pharmacy and Pharmaceutical Sciences for cannabis extraction, isolation, formulation, and analytical testing.
ON BEHALF OF THE BOARD
“Hugh Rogers”
Hugh Rogers, CEO and Director
Investor Inquiries:
Mr. Knox Henderson
Tel: 604-551-2360
info@xphyto.com
www.xphyto.com
Forward looking statements
This news release includes statements containing forward-looking information within the meaning of applicable Canadian securities law (“forward-looking statements”). Forward-looking statements are frequently characterized by words such as “develop”, “plan”, “continue”, “expect”, “project”, “intend”, “believe”, “anticipate”, “estimate”, “potential”, “propose” and other similar words, or statements that certain events or conditions “may” or “will” occur, and in this release include the statement regarding the Company’s goal of building a successful diagnostic, drug delivery, and medical cannabis company. Forward-looking statements are only predictions based on the opinions and estimates of management at the date the statements are made and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements, including: that the Company may not succeed in developing a commercial product; that the sale of products may not be a viable business; that the Company may be unable to scale its business; product liability risks; product regulatory risk; general economic conditions; adverse industry events; future legislative and regulatory developments; inability to access sufficient capital from internal and external sources, and/or inability to access sufficient capital on favourable terms; currency risks; competition; international risks; and other risks beyond the Company’s control. The Company is under no obligation, and expressly disclaims any intention or obligation, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as expressly required by applicable law.
Neither the CSE nor its Market Regulator (as that term is defined in the policies of the CSE) accepts responsibility for the adequacy or accuracy of this news release.
[1] Wölfel, R., Corman, V.M., Guggemos, W. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465-469 (2020).
[2] He, X., Lau, E.H.Y., Wu, P. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 26, 672-675 (2020).
[3] To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 20(5), 565-574 (2020).
SOURCE: XPhyto Therapeutics Corp.
View source version on accesswire.com:
https://www.accesswire.com/600935/XPhyto-Provides-Update-on-Covid-19-Rapid-Test-Commercialization
