Theralase’s 5th Annual Medical and Scientific Advisory Board Meeting Advances Phase II Non-Muscle Invasive Bladder Cancer Clinical Study
TORONTO, ON / ACCESSWIRE / May 13, 2019 / Theralase® Technologies Inc. (“Theralase” or the “Company“) (TSXV: TLT) (OTCQB: TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated PhotoDynamic Compounds (“PDC“) and their associated drug formulations intended to safely and effectively destroy various cancers announced that Theralase recently met with its Medical and Scientific Advisory Board (“MSAB“), as well as potential Principal Investigators (“PIs“), who are interested in participating in the Phase II Non-Muscle Invasive Bladder Cancer (“NMIBC“) clinical study (“ACT-NMIBC“).
The meeting focused primarily on the presentation of the clinical endpoints achieved in the successfully completed Phase Ib NMIBC clinical study, as well as a discussion on the design and FDA regulatory requirements pertaining to the ACT-NMIBC.
The FDA provided guidance to the industry in February 2018, entitled, “BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment Guidance for Industry“.
In the guidance, the FDA stated that, “In BCG-unresponsive NMIBC, a single-arm clinical trial with complete response rate and duration of response as the primary endpoint can provide primary evidence of effectiveness to support a marketing application.” 1
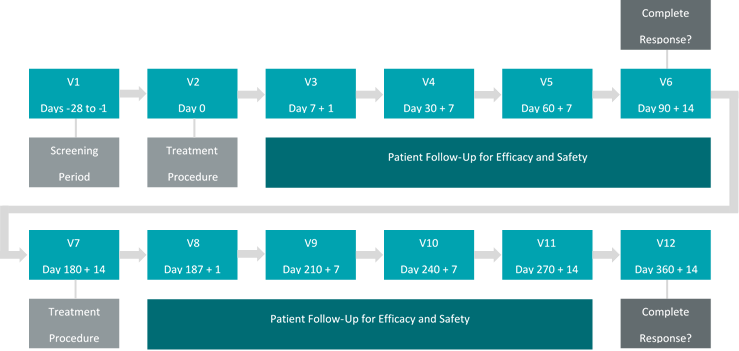
Theralase’s primary question to be discussed with the FDA during a pre-Investigational New Drug (“pre-IND“) conference call to be held in early 3Q2019 will be “If Theralase’s ACT-NMIBC demonstrates a Complete Response (“CR”) at 90 days and a duration of that CR of 30% or more at 360 days post primary treatment, would this be sufficient to support a marketing application in the opinion of the FDA?“
The PIs at the meeting consisting of leading Canadian, US and international uro-oncologists, were presented with the opportunity to learn more about Theralase’s TLD-1433-based PhotoDynamic Therapy (“PDT“) and its application to BCG-Unresponsive NMIBC.
Dr. Shawn Shirazi, CEO-Drug Division, Theralase stated, “Theralase stakeholders should be excited about the ability of the Company to execute on its strategic objectives, from successfully completing the Phase Ib NMIBC clinical study to the commencement of the ACT-NMIBC study. The Company is focusing on opening Canadian, US and international study sites throughout Q2 2019 and Q1 2020 with the PIs that attended the 5th annual MSAB meeting. The overall response of the PIs was extremely encouraging, with the MSAB and PIs recognizing the hard work and dedication of the Theralase team in the execution of its clinical development strategy to advance a viable treatment to potential commercialization for BCG-Unresponsive NMIBC.”
About Phase Ib Study
Theralase’s Phase Ib NMIBC clinical study successfully achieved the: primary endpoint of safety and tolerability, secondary endpoint of pharmacokinetics (movement and exit of TLD-1433 within the body) and exploratory endpoint of efficacy. The study results have shown a strong efficacy signal with a 66% CR in the Therapeutic Dose Group (0.70 mg/cm2) after only a single PDT treatment, with patients 5 and 6 demonstrating no presence, recurrence or progression of the disease, 360 days post treatment.
About Phase II Study
The ACT-NMIBC clinical study will utilize the Therapeutic Dose (0.70 mg/cm2) of TLD-1433 and will focus on the treatment of approximately 100 to 125 BCG-Unresponsive NMIBC patients in approximately 20 clinical study sites located in Canada, the US and internationally, with a primary endpoint of efficacy and a secondary endpoint of safety.
The primary endpoint will be evaluated by:
CR in patients with Carcinoma In-Situ (“CIS“) with or without resected papillary disease at 90 days post-treatment with a duration of CR evaluated at 360 days post-treatment.
Patient CR is defined as at least one of the following:
1) Negative cystoscopy and negative (including atypical) urine cytology
2) Positive cystoscopy with biopsy-proven benign or low-grade NMIBC
3) Negative cystoscopy with malignant urine cytology, if cancer is found in the upper tract or prostatic urethra and random bladder biopsies are negative
The secondary endpoint will be evaluated by:
Incidence and severity of Adverse Events (“AEs“) Grade 4 or higher that do not resolve within 360 days post-treatment; whereby:
Grade 1 = Mild, Grade 2 = Moderate, Grade 3 = Severe, Grade 4 = Life-threatening or disabling, Grade 5 = Death
Proposed Clinical Treatment Plan:

About Theralase’s MSAB
The MSAB is comprised of scientific and clinical professionals in PDT and bladder cancer and have been retained by the Company to provide strategic guidance on the research, development and commercialization of the Company’s TLD-1433-based PDT technology in the treatment of patients inflicted with BCG-Unresponsive NMIBC.
About Theralase® Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds and their associated drug formulations intended to safely and effectively destroy various cancers.
Additional information is available at www.theralase.com and www.sedar.com
This news release contains “forward-looking statements” which reflect the current expectations of management of the Company’s future growth, results of operations, performance and business prospects and opportunities. Such statements include, but are not limited to, statements regarding the Company’s proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Wherever possible, words such as “may“, “would“, “could“, “should“, “will“, “anticipate“, “believe“, “plan“, “expect“, “intend“, “estimate“, “potential for” and similar expressions have been used to identify these forward-looking statements. These statements reflect management’s current beliefs with respect to future events and are based on information currently available to management. Forward-looking statements involve significant risks, uncertainties and assumptions including with respect to the ability of the Company to: adequately fund, secure the requisite regulatory approvals to commence and successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its development plans. Many factors could cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements; including, without limitation, those listed in the filings made by the Company with the Canadian securities regulatory authorities (which may be viewed at www.sedar.com). Should one or more of these risks or uncertainties materialize or should assumptions underlying the forward looking statements prove incorrect, actual results, performance or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. The Company disclaims any intention or obligation to revise forward-looking statements whether as a result of new information, future developments or otherwise except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchanges) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
1.866.THE.LASE (843-5273) x 304
416.699.LASE (5273) x 304
Amelia Tudo, Investor Relations Coordinator
atudo@theralase.com
www.theralase.com
1https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM529600.pdf
SOURCE: Theralase® Technologies Inc.
View source version on accesswire.com:
https://www.accesswire.com/545049/Theralases-5th-Annual-Medical-and-Scientific-Advisory-Board-Meeting-Advances-Phase-II-Non-Muscle-Invasive-Bladder-Cancer-Clinical-Study
