FDA Clearance of the Uro-G Completes UroViu Corporation’s Suite of Portable, Single-Use, Disposable Cystoscopes
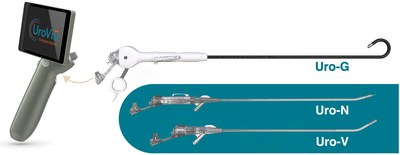
LOS ALTOS, Calif., May 24, 2021 /PRNewswire/ — UroViu Corporation, the developer of a suite of portable, self-contained and versatile single-use cystoscopy solutions, has received clearance from the U.S. Food and Drug Administration to market its third device: Uro-G, a flexible single-use cystoscope with a fully deflectable tip that enables physicians to perform interventional and diagnostic urologic procedures in their clinics conveniently in any room, anytime, without reprocessing. This allows for practices to expand their cystoscopic capabilities and throughput without capital investments or service contracts. Typically, the per-procedure cost of owning and using a UroViu device is lower than for traditional reusable platforms.
“Early adopters of UroViu’s pioneering technology will value the practicality of this safe, user-friendly and effective option, both for practice and patients,” said Jed Kaminetsky M.D., clinical assistant professor of urology at the NYU Grossman School of Medicine and a medical director of Manhattan Medical Research. “In particular, we find the off-the-shelf availability of Uro-G to be extremely efficient and convenient in performing outpatient diagnostic procedures and stent removals.”
Roger R. Dmochowski, M.D., professor of urologic surgery at Vanderbilt University Medical Center stated that “We are encouraged at the prospect of employing single-use devices that provide the same or better results for our patients without the heavy investment of time and capital in reprocessing”. He also appreciates the opportunity to avoid the risk of cross-contamination associated with reusable cannulas — which, in rare cases, have been linked with drug-resistant infections and even death.1 He added that the nimbleness, cost savings, and excellent safety profile of the Uro-G Cystoscope can result in fewer delays in the scheduling of diagnostic procedures, a crucial step in caring for patients.
UroViu will be introducing its full product range at this year’s American Urological Association (AUA) annual meeting on Sep 9-13th in Las Vegas, NV. Contact us for further information.
References:
- FDA is Investigating Reports of Infections Associated with Reprocessed Urological Endoscopes. FDA website. https://www.fda.gov/news-events/press-announcements/fda-investigating-reports-infections-associated-reprocessed-urological-endoscopes.Updated April 1, 2021. Accessed May 18, 2021.
About UroViu
UroViu Corporation’s mission is to revolutionize the cystoscopy platform for improved patient care and efficiency and has operations in California and Washington and production facilities in Asia. UroViu is the developer of the always ready cystoscopy platform, the most portable, self-contained and versatile single-use cystoscopy solution on the market. The intuitive portable platform and product suite are protected by a portfolio of 13 granted and many more pending patents. UroViu’s vision is to simplify the scope of patient care.
Media Contacts:
Sebastien Cadet
UroViu Corporation
Marketing@uroviu.com
View original content to download multimedia:http://www.prnewswire.com/news-releases/fda-clearance-of-the-uro-g-completes-uroviu-corporations-suite-of-portable-single-use-disposable-cystoscopes-301297271.html
SOURCE UroViu

