ImpediMed Announces Next Generation of SOZO® Digital Health Platform Software
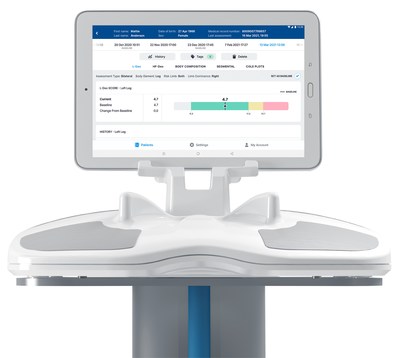
CARLSBAD, Calif., June 7, 2021 /PRNewswire/ — ImpediMed Limited (ASX:IPD), a global medical technology company using bioimpedance spectroscopy (BIS) to generate powerful data to improve patient health, today announced the release of the next generation software for the SOZO® Digital Health Platform. This software release, designated as Version 4.0, adds an intuitive redesign, enhanced security and privacy measures, and personalized data sets to provide clinicians with in-depth, actionable information to maximize patient health.
“The latest update to the SOZO Digital Health Platform provides clinicians with even more power to monitor patient health and make crucial clinical care decisions. Version 4.0 allows care teams to generate personalized patient datasets, compare previous measurements with current ones, and add notes and tags to patient profiles,” said Richard Carreon, Managing Director and CEO of ImpediMed. “At ImpediMed, our mission is to not only develop technologies to help clinicians in monitoring and determining the best course of treatment for their patients, but to advance and adapt our technologies to better suit the needs of the clinicians and their patients.”
The SOZO Digital Health Platform has gained broad adoption worldwide. Over 700 devices are in use by large and small healthcare systems as well as by AstraZeneca in support of clinical trials. The software improvements in this release are supported by clinical experience from over 250,000 SOZO patient tests and extensive human factors testing. SOZO’s cloud-based software solution provides customers with immediate access to updates, which take only minutes to complete.
The SOZO Digital Health Platform Version 4.0 software includes advancements such as a major redesign of the User Interface, making the software even more intuitive and easier to use for clinicians managing multiple patients across a variety of conditions. The visual display incorporates further customization, enabling clinicians to tag measurements and make patient notes supporting clinical decision making while assisting clinicians in the timely retrieval of critical information.
The upgraded software also features new tools to monitor patients’ body compositions. Novel reference ranges have been added to the body composition analysis, combining tissue and fluid analyses into one. An update to the segmental analysis implements the SOZO Digital Health Platform’s new license for measuring composition of the arms and legs. Version 4.0 also includes enhanced privacy and security features such as a hidden patient list, Multi Factor Authentication (MFA), and Single Sign On (SSO) to protect patient data.
The SOZO® device is a noninvasive monitoring tool using bioimpedance spectroscopy (BIS) technology to deliver a precise snapshot of fluid status and tissue composition in less than 30 seconds with 256 unique data points. The FDA-cleared, CE-marked and ARTG-listed digital health platform assists clinicians in the early detection of secondary lymphedema and measures fluid status for patients living with heart failure. It provides a patient’s L-Dex® score, the only non-invasive, reliable, validated tool to help clinicians identify subclinical lymphedema.
About ImpediMed
Founded in Brisbane, Australia, with U.S. headquarters and European operations, ImpediMed is a global medical technology company using bioimpedance spectroscopy (BIS) to generate powerful data to maximize patient health. ImpediMed produces a family of FDA cleared and CE Marked medical devices, including SOZO® for multiple indications, including heart failure, lymphedema, and protein calorie malnutrition sold in select markets globally. Visit www.impedimed.com.
About SOZO Digital Health Platform
SOZO, the world’s most advanced, noninvasive bioimpedance spectroscopy (BIS) device, delivers a precise snapshot of fluid status and tissue composition in less than 30 seconds. Using ImpediMed’s BIS technology, SOZO measures 256 unique data points over a wide spectrum of frequencies from 3 kHz to 1000 kHz. Results are available immediately online for easy data access and sharing across an entire healthcare system. The FDA-cleared, CE-marked and ARTG-listed digital health platform aids in the early detection of secondary lymphedema, provides fluid status for patients living with heart failure, and can be used to monitor and maintain overall health – all on a single device.
For more information, visit: https://www.impedimed.com/products/sozo/?utm_source=sozo_4-0_launch&utm_medium=us_news_release&utm_term=&utm_content=textlink&utm_name=news_4-0_2021
About SOZO L-Dex Analysis for Lymphedema
The SOZO fluid analysis for lymphedema provides the L-Dex score to measure fluid build-up in a limb at risk for lymphedema. By identifying the patient’s baseline L-Dex score before cancer treatment and then measuring it at regular intervals post-surgery, healthcare professionals can accurately monitor patient progress and offer appropriate education and intervention to prevent the progression of lymphedema. One-year interim results from the PREVENT Trial showed that this early detection combined with at-home intervention using standard compression therapy can reduce the progression of lymphedema by 95%. Use of L-Dex and BIS for the clinical assessment of lymphedema is recommended in multiple clinical practice guidelines, including the American Physical Therapy Association, National Lymphedema Network, and Lymphatic Education & Research Network Centers of Excellence program.
For more information, visit: https://www.impedimed.com/products/tissue-fluid-applications/l-dex-analysis-for-lymphedema/?utm_source=sozo_4-0_launch&utm_medium=us_news_release&utm_term=&utm_content=textlink&utm_name=news_4-0_2021
U.S. Media Contacts:
David Schull or Nic Johnson
Russo Partners
(858) 717-2310
David.schull@russopartnersllc.com
Nic.johnson@russopartnersllc.com
View original content to download multimedia:http://www.prnewswire.com/news-releases/impedimed-announces-next-generation-of-sozo-digital-health-platform-software-301306756.html
SOURCE ImpediMed Limited
