Advantis Medical Imaging Receives FDA Clearance for Brain MRI Analysis Software
ATHENS, Greece, Nov. 16, 2021 /PRNewswire/ — Advantis Medical Imaging, a pioneering manufacturer of cloud-based medical imaging software solutions, today announced its first 510(k) clearance from the U.S. Food and Drug Administration (FDA). The clearance is for Brainance MD™, a groundbreaking and all-in-one neuroimaging platform for the analysis of brain MRI exams.
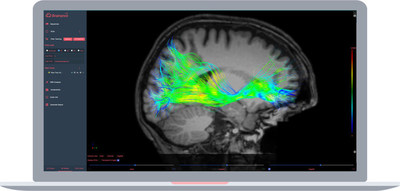
Accessible from a web browser, Brainance MD™ is designed to enable the display, processing, and analysis of brain MR images, including diffusion tensor imaging (DTI), dynamic susceptibility contrast (DSC) perfusion, and functional MRI (fMRI) – all in a unified and highly intuitive user interface. The fully DICOM compliant application is provided as a Software as a Service (SaaS) and offers analysis and visualization capabilities of dynamic MRI data of the brain, presenting the results in a clinically useful context and further assisting clinicians during the diagnostic workflow of brain MRI exams.
“Advanced brain MRI processing techniques which were previously regarded as more research-oriented due to their scientific complexity and time-consuming manual processing needs are now available to clinicians in an all-in-one, automated and highly intuitive software.” said Zoi Giavri, CEO & CSO. “Our solution eliminates any unnecessary manual work, embraces remote access and collaborative exam review, enhances standardisation and reduces operating overhead for clinical organizations through its zero-footprint nature and optimized processing pipelines.”
“Today’s modern radiology departments are in constant search of highly reliable, automation-driven and user-friendly software tools which can alleviate their rapidly growing workloads and assist them in focusing more on clinical findings.” said Paris Ziogkas, COO & Head of Business Development. “This FDA clearance is a pivotal milestone for our company and marks the beginning of a series of product development announcements as we are expanding to other human organs aiming to offer a more holistic imaging suite, which covers a wide spectrum of medical image processing needs and pathologies.”
Brainance MD™ is currently in use in Europe as a CE Marked device where numerous clinical organizations have integrated it in their daily clinical brain MRI processing workflows. With this first FDA clearance the company aims to collaborate with U.S. clinicians by assisting them to focus on clinical findings and save valuable time.
About Advantis Medical Imaging
Advantis Medical Imaging was founded in 2016 and has offices in Eindhoven, The Netherlands and Athens, Greece. The company is on a mission to make medical image analysis more quantitative, accessible and automated through AI and cloud technology. Advantis’ activity and growth revolve around its central value which governs its every action: To continuously strive for progress and invent new and innovative products which make a real impact for its customers and their patients.
Source: Advantis Medical Imaging
Mr. Paris Ziogkas
Email: info@advantis.io
Website: https://advantis.io/
Linkedin: https://www.linkedin.com/company/advantis-medical-imaging
Tel: +31 858882140
View original content to download multimedia:https://www.prnewswire.com/news-releases/advantis-medical-imaging-receives-fda-clearance-for-brain-mri-analysis-software-301424497.html
SOURCE Advantis Medical Imaging

