Premia Spine Appoints Three New Board Advisors

World-renowned Spine Surgeon, Successful Medical Device Executives Named in Preparation for U.S. Commercial Launch of the TOPS System
NORWALK, Conn.–(BUSINESS WIRE)–#lumbarspinalstenosis—Premia Spine, a medical technology company poised to improve the way debilitating chronic leg and back pain is surgically treated, today announced the appointment of three new advisors to its board of directors. Bringing decades of clinical, business and medical device innovation experience to Premia Spine, the new appointees will provide critical input supporting the commercial launch of the company’s TOPS™ System. TOPS is a motion preserving solution for 350,000 patients worldwide who suffer from degenerative spondylolisthesis and lumbar spinal stenosis who either undergo fusion surgery each year or persevere with these debilitating diseases for fear of rigid spinal fixation.
Stephen Hochschuler, MD is founder and chairman emeritus of the Texas Back Institute. A renowned orthopedic surgeon, he is a serial entrepreneur and innovator in the field of spinal surgery. Dr. Hochschuler is past president (2006-2007) and founding member of the International Society for the Advancement of Spine Surgery (ISASS), chairman of the board of Innovative Spinal Technologies of Plano, and founder of several medical-related companies. Dr. Hochschuler is a member of numerous national and international professional organizations and is published in a wide range of professional journals.
Peter Wehrly is a C-suite medical device industry executive with extensive expertise in delivering top line growth and operational efficiencies. A highly successful industry insider, Mr. Wehrly is chief executive officer (CEO) at Vivex Biologics. Previously he served as CEO at Medingo, which was acquired by Roche, and as President and CEO of PQ Bypass, which was acquired by Endologix. He has also held executive leadership positions at Medtronic, DePuy Orthopaedics and Covidien. Mr. Wehrly excels at bringing new technologies to market, securing profitable strategic alliances, contracts and agreements. He also has an extensive record in private, public and community board involvement.
Nicholas Pachuda, DPM is a medical device, biotech, and orthopedics innovator, investor and advisor. As former worldwide vice president of external innovation within the Medical Device Group at Johnson & Johnson, Dr. Pachuda held sweeping responsibilities including leading global strategic innovation, business development, portfolio and channel development, and commercial business unit and new venture incubation. He led the Johnson & Johnson JPOD startup incubator in Philadelphia. He was a cofounder of the extremities division at Arthrex. He was part of the leadership team at Small Bone Innovations that was acquired by Stryker, and global VP of marketing at Scient’x Spine that was acquired by Alphatec.
“I have joined as a Board Advisor to Premia Spine because I see the potential for the TOPS System to change the standard of care for treating patients with spinal stenosis and degenerative spondylolisthesis,” said Dr. Hochschuler. “After observing the technology for many years, reviewing the clinical trial design and its interim results, I believe Premia Spine could make a positive impact on patients and spine surgery. I look forward to working with Premia Spine’s Board and management team to help bring TOPS to market.”
Premia Spine CEO Ron Sacher added, “I am gratified that this prestigious roster of clinical, innovation and business experts recognize the potential of the TOPS System and want to contribute to its success. Their strategic contributions are already being realized as we continue with the clinical study of TOPS and prepare for commercial launch in 2022. I am confident that their insights will help position us to be a leader in helping those suffering from spinal stenosis and degenerative spondylolisthesis.”
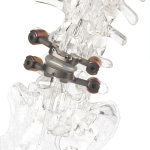
The first and only facet replacement device which is inserted into the lumbar vertebral joint to stabilize the spine, TOPS was developed to provide mobility, stability and durability after decompression for patients with lumbar spinal stenosis and degenerative spondylolisthesis. TOPS recently won Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) and is currently the subject of a pivotal clinical trial under an investigational device exemption from the FDA.
Spinal stenosis and degenerative spondylolisthesis are painful, debilitating and highly prevalent conditions that impact over 100 million people globally1. An estimated 350,000 people undergo lumbar spinal fusion each year for these conditions2, representing a $2 billion annual addressable global market.
About Premia Spine
Premia Spine, a medical technology company, aims to improve the lives of chronic leg and back pain patients with its TOPS™ System. TOPS is designed to provide lasting mobility, stability and durability to patients with lumbar spinal stenosis, degenerative spondylolisthesis and related spinal conditions. For more information, visit premiaspine.com.
____________________
1 Ravindra, V. M., et al. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Global Spine Journal: 2018, Vol 8, Issue 8. https://journals.sagepub.com/doi/full/10.1177/2192568218770769
2 2019 Spinal Surgery Update. Orthopedic Network News: Volume 30, Number 4, October 2019.
Contacts
Media Contact:
Joe Duraes
Pazanga Health Communications
jduraes@pazangahealth.com
(917) 687-6419