Halberd CEO Letter: 2021 Year-End & 2022 Outlook
Jackson Center, Pennsylvania–(Newsfile Corp. – January 25, 2022) – Greetings From The CEO And Staff At HALBERD CORPORATION (OTC Pink: HALB)!
First of all, I want to wish all of our shareholders and constituents a Very Healthy, Happy and Prosperous New Year!
2021 OVERVIEW AND ACHIEVEMENTS
- Halberd continued its R&D strategy to focus on biomedical areas where:
- There is a huge worldwide demand for cures to long standing diseases;
- There is no effective cure available;
- Big Pharma had either failed to meet that demand or had effectively abandoned the effort; and
- Halberd’s patent position insured high potential of technical and market success.
- Halberd chose neurodegenerative diseases as its primary focus, since there are approximately 36 million people cumulatively suffering from PTSD/ CTE, Alzheimer’s Disease, Lou Gehrig’s Disease, Parkinson’s Disease, and Epilepsy in the USA alone. The total worldwide is proportionately much higher.
- Halberd’s corporate strategy was defined as focusing on completing successful proof-of-concept testing of its patented extracorporeal treatment methodology and subsequently seeking to form joint ventures with Big Pharma organizations and/or university hospital complexes worldwide to secure adequate funding for completion of development testing and regulatory approval prior to market introduction.
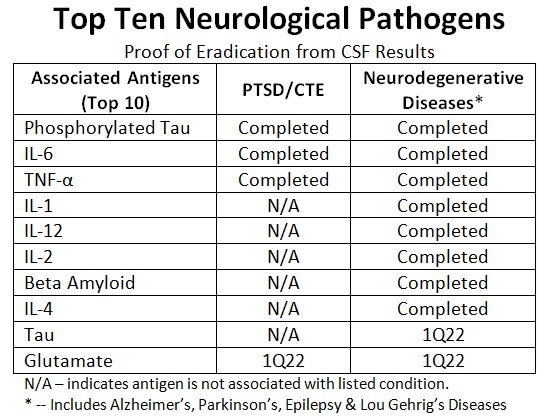
- Halberd identified three antigens (Tau, Phosphorylated Tau, & Beta Amyloid), six inflammatory cytokines (IL-1, IL-2, IL-4, IL-6, IL-12, and TNF-Alpha) and one excitatory neurotransmitter (Glutamate) as linked to neurodegenerative diseases; later classified as Halberd’s “Top Ten Target Antigens.”
Top Ten Neurological Pathogens
To view an enhanced version of this graphic, please visit:
https://healthtechnologynet.com/wp-content/uploads/2022/01/111450_4a918ab6a49d9889_001full.jpg
-
Halberd developed a proprietary process to conjoin metallic nanoparticles to proprietary antibodies, forming a two-part metallic moiety. After joining the metallic moiety to the antigens, eradication of the antibody-antigen complex is accomplished via laser emissive energy or radio frequency exposure.
-
Halberd successfully completed work on eradicating 100% of all eight of the Top Ten target antigens tested so far linked to neurodegenerative diseases. Eradication of Halberd’s remaining two Target Antigens has been delayed by lack of availability of commercial reagent supplies from worldwide sources.
-
The principal competitive Alzheimer’s drug treatment available today eliminates 5-15% of Beta Amyloid after one month of treatment costing over $50,000 per year. Halberd’s extracorporeal process eradicates 100% within just 10 minutes.
-
Meetings have been held with representatives from the NFL Retired Players Association (NFLPA) to develop a strategy for presenting our results to NFL management to seek their support for ongoing research toward developing a cure for PTSD/CTE. Meetings have not yet been established with NFL management.
-
Halberd added an expert Nephrologist to its staff in anticipation of blood-borne tests, slated to get underway after completion of testing in cerebral spinal fluid (CSF).
-
One issued patent has been added this year to Halberd’s IP for the extracorporeal treatment of disease, bringing the total to three related issued patents. Additionally, Halberd added several provisional patent applications bringing that total to twenty. Halberd is working hard to protect its intellectual property to prevent potential theft and/or duplication by competitors.
-
Halberd Corp. commenced an independent audit in the fourth quarter to work towards its planned FORM-10 application to achieve fully reporting status with the SEC under the Exchange Act of 1934.
-
Halberd has been approved as a governmental contractor making it eligible to participate in development grants, and is currently awaiting a decision on such a potential large government grant associated with PTSD/CTE.
FORWARD GOALS
-
Halberd’s on-going independent audit is anticipated to be completed within Q1 2022.
-
Efforts are underway on filing Halberd’s FORM-10 contingent on the completion of its independent audit leading to OTCQX exchange up-list by or within Q2 2022.
-
Completion of the Top Ten Target Antigen elimination from CSF is expected to be completed within Q1 2022.
-
Establish and execute meetings with the NFL and possibly the NCAA to seek endorsement for Halberd’s PTSD/CTE research.
-
Halberd plans on further expanding brand recognition and outreach by communicating with investors and the public via appearances on New-To-The-Street, Money TV, Stock Day Podcast along with periodic press releases and email blasts to our subscribers to provide visibility on our progress.
-
Discussions have commenced and are in progress with a large medical university to develop potential cures for several neurodegenerative diseases with potential independent validation within the year 2022. We plan on sharing the name of this university once an agreement is established.
-
We are looking into conducting initial discussions with an identified pharmaceutical company for potential partnering arrangements.
-
The next steps in Halberd’s R&D program include eradication of the “Top Ten Target Antigens” from blood elements (serum and plasma).
-
Plans are underway for generating scientific articles for publication within Q1 2022. The material will be carefully screened for patentable content prior to publication to protect our intellectual property and achievements.
-
Halberd is currently searching for an infectious disease specialist to be added to its company staff within Q1 2022 to assist in developing and testing cures for infectious diseases which have the potential of being treated with Halberd’s proprietary technology.
-
Halberd plans to expand its over-the-counter (OTC) supplement product line with a brain function support product. The brain function support product is currently being formulated and designed. We plan on sharing updates as we progress.
-
Halberd will continue its efforts in developing relationships toward placing and marketing its nutraceutical line products in both Central and South America to increase both brand recognition and revenue.
-
Conclude current ongoing talks with a major university to acquire related abandoned intellectual property that could expand Halberd’s value significantly.
-
Continue to protect Halberd’s intellectual property by timely filing of patent applications.
SUMMARY
In summary, the Halberd team is expanding its technical capabilities and has exhibited solid progress in 2021. We believe our strategy going forward is exciting and will lead to success for the company and our stockholders/shareholders in 2022 and beyond.
We look forward to a healthy, happy and prosperous 2022 for all.
Sincerely,
William A. Hartman
Chairman, President & CEO
HALBERD CORPORATION
w.hartman@halberdcorporation.com;
support@halberdcorporation.com
www.halberdcorporation.com
Twitter:@HalberdC
About Halberd Corporation.
Halberd Corporation (the “Company”) is an innovative biotech company focused on developing treatments through its acquired exclusive licenses, patents, and provisional patent applications for illnesses including and limited to; PTSD, CTE, cancer, blood-borne, and cerebrospinal fluid related diseases. To learn more, please visit https://halberdcorporation.com.
Halberd has obtained exclusive worldwide rights to three issued patents and has filed twenty related provisional, PCT, or utility patent applications to enhance its value to its stockholders and to attract the interests of potential development partners.
Safe Harbor Notice
Certain statements contained herein are “forward-looking statements” (as defined in the Private Securities Litigation Reform Act of 1995). The Companies caution that statements, and assumptions made in this news release constitute forward-looking statements and makes no guarantee of future performance. Forward-looking statements are based on estimates and opinions of management at the time statements are made. These statements may address issues that involve significant risks, uncertainties, estimates made by management. Actual results could differ materially from current projections or implied results. The Companies undertake no obligation to revise these statements following the date of this news release.
Investor caution/added risk for investors in companies claiming involvement in COVID-19 initiatives –
On April 8, 2020, SEC Chairman Jay Clayton and William Hinman, the Director of the Division of Corporation Finance, issued a joint public statement on the importance of disclosure during the COVID-19 crisis.
The SEC and Self-Regulatory Organizations are targeting public companies that claim to have products, treatment or other strategies with regard to COVID-19.
The ultimate impact of the COVID-19 pandemic on the Company’s operations is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak. Additionally, new information may emerge concerning the severity of the COVID-19 pandemic, and any additional preventative and protective actions that governments, or the Company, may direct, which may result in an extended period of continued business disruption, reduced customer traffic and reduced operations. Any resulting financial impact cannot be reasonably estimated at this time.
We further caution investors that our primary focus and goal is to battle this pandemic for the good of the world. As such, it is possible that we may find it necessary to make disclosures which are consistent with that goal, but which may be adverse to the pecuniary interests of the Company and of its shareholders.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/111450
