EyeControl Expands R&D Program with EyeControl-Pro for Delirium
Company to exhibit and share early clinical results at the American Delirium Society Conference, June 12-14, Indianapolis, IN
A multi-center, international clinical trial to investigate the safety and efficacy of EyeControl-Pro for Delirium reduction is underway
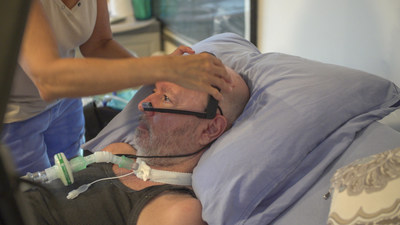
TEL AVIV, Israel, June 13, 2022 /PRNewswire/ — EyeControl is developing the EyeControl-Pro, an AI-based, smart, screenless and wearable, eye-tracking communication solution for Delirium reduction. The company will display its new investigational device, EyeControl-Pro, at the American Delirium Society Conference (ADS), June 12-14, Indianapolis, IN.
EyeControl-Pro is being designed to enable bi-directional remote connectivity and communication between the patient’s headset, the medical team and family members.
EyeControl CEO Mr. Or Retzkin said, “Our intention is to effectively empower the up to 80% of ICU patients experiencing Delirium to communicate with their medical support team, family and friends, center and regain control of reality. Delirium costs the US healthcare system up to $152B annually. We’ll speak about our vision and plans for Delirium management at the ADS conference.”
EyeControl will also share early data from its phase I clinical trial in Israel at Beilinson Hospital’s ICU, where preliminary results include decreases in patient errors in the Confusion Assessment Method for the ICU (CAM-ICU), a validated Delirium screening tool, as well as improvements in Lowenstein Communication Scale (LCS) scores and patient engagement with hospital staff.
EyeControl-Pro is also currently being evaluated in a multi-center, international clinical trial to investigate the safety and efficacy of the device for Delirium reduction.
Delirium is an acute mental disturbance characterized by confused thinking and disrupted attention sometimes accompanied by hallucinations; it can be brought on by anxiety, isolation and cognitive decline, which arises from lack of communication and interpersonal interactions.
About EyeControl
EyeControl has developed the first AI-based, smart, screenless and wearable, eye-tracking communication solution intended to address the unmet healthcare needs of patients, who cannot speak. The company was founded in 2016 by individuals who share unique personal connections to ALS and Locked-in patients, in an effort to restore their communication needs.
EyeControl-Med provides comprehensive, round-the-clock, bi-directional connectivity between acute care patients with communication difficulties, their families, and medical teams. This increases patient engagement and emotional wellbeing in ICUs, long-term acute care facilities, and rehabilitation centers. The product is FDA 510(k)-exempt, CE marked, ISO certified, and HIPAA/GDPR compliant.
EyeControl-Pro, based on the same communication platform, is an investigational device being developed to treat those experiencing Delirium. Clinical trials investigating its safety and efficacy in reducing Delirium are currently underway in Israel and the US. EyeControl-Pro for Delirium reduction is for investigational use only and has not been cleared or approved by the US FDA. The device is not available for sale in the US.
Investors include Connecticut Innovations, certain VC funds, angels, the European Innovation Council (EIC) and EIC Fund, the Israel Innovation Authority and Israel Ministry of Economy.
For more information please visit: www.eyecontrol.co.il.
Photo – https://healthtechnologynet.com/wp-content/uploads/2022/06/EyeControl.jpg
Press Contact
Marjie Hadad
General Manager
Must Have Communication
917-790-1178
marjie@mhc-pr.com
View original content to download multimedia:https://www.prnewswire.com/news-releases/eyecontrol-expands-rd-program-with-eyecontrol-pro-for-delirium-301566624.html
SOURCE EyeControl
