Elixia Health Enters Phase I Clinical Trials Market

Tampa-based site expands study capabilities and patient comfort
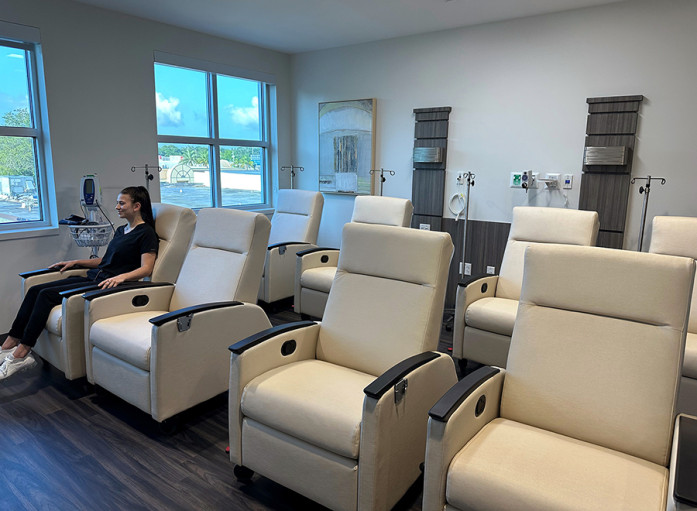
HOLLYWOOD, FL / ACCESSWIRE / August 10, 2023 / Elixia Health, a leading provider of clinical trials management, analysis and patient recruitment services, announces the opening of its Phase I Unit for clinical research, which began operations on June 1. The 40-bed inpatient private research site is located in Tampa, Fla., near a Level 1 trauma center hospital. The facility provides in-house 24-hour medical coverage and comfortable accommodations for study participants, including those making overnight stays.
Elixia Health’s Chief Scientific Officer, Dr. Harry Alcorn, is serving as administrator of the Phase I Unit. With over 20 years of Phase I experience, Dr. Alcorn has been the principal investigator on more than 150 protocols and sub-investigator on more than 250 protocols. He designed and created the U.S. Renal Network, the first network of Phase I renal research sites in the country.
“Our new unit, equipped with cutting-edge facilities and a seasoned team, is precisely focused on the underserved areas of renal and hepatic patient trials,” said Dr. Alcorn. “Our entry into the Phase I market underlines Elixia’s intent to catalyze breakthroughs that may pave the way for new, FDA-approved medications.”
The Elixia Health Phase I Unit’s clinical trial capabilities include pharmacokinetics, pharmacodynamics, bioavailability/bioequivalence, dose-response, first-time-in-human safety/tolerance, renal studies, IV infusion studies, and more. The site’s research areas cover a number of specialty populations (renal, hepatic, cardiovascular, diabetic, and gastrointestinal patients), as well as healthy males, females, and seniors.
The Phase I Unit is equipped with full pharmacy services, 12-lead ECG, refrigerated centrifuges, secure ultra-low temperature freezers, and backup generator electrical supply. Patient amenities include comfortable exam rooms, a recreation area, and private overnight rooms furnished with TVs and tablets with wifi access.
“This step is more than an expansion; it’s a clear message that Elixia is prepared to address and resolve the challenges of today’s clinical research needs,” added Jim Crissy, the company’s Chief Operating Officer. “By combining our cutting-edge infrastructure with Dr. Harry Alcorn’s esteemed leadership, we aim to set new standards in Phase I clinical research.”
# # #
About Elixia Health
Founded in 2019, Elixia Health is an innovator in patient recruitment and clinical trials management and analysis for three targeted therapeutic areas: cardiology/nephrology, behavioral health and infectious disease. The company holds the record for enrolling the most patients in the shortest amount of time for any global trial in the last 50 years. Elixia Health’s breakthrough approach helps drug developers not only meet milestones with record speed and agility, but enroll and retain the right patients, trial after trial. Learn more at elixiahealth.com.
Contact Information:
Sam Searcy
Chief Commercial Officer
ssearcy@elixiacrc.com
919-904-0460
SOURCE: Elixia Health
View source version on accesswire.com:
https://www.accesswire.com/773171/Elixia-Health-Enters-Phase-I-Clinical-Trials-Market
