FDA Clearance of VerTouch™ from IntuiTap Medical Could Revolutionize Epidurals

12.7 million Americans undergo an epidural, spinal block or lumbar puncture annually.
HOUSTON–(BUSINESS WIRE)–#epidurals–IntuiTap Medical proudly announces FDA clearance of their cutting-edge medical device, VerTouch. VerTouch eliminates the guesswork from spinal punctures making them more accurate and consistent. This milestone achievement for IntuiTap signifies a significant leap forward in the company’s mission to set a new standard of care for the millions of Americans who undergo these procedures annually.
FDA clearance validates the safety and effectiveness of IntuiTap’s novel device, VerTouch, which has undergone rigorous testing at medical institutions across the United States including Northwestern Memorial Hospital and the Texas Medical Center.
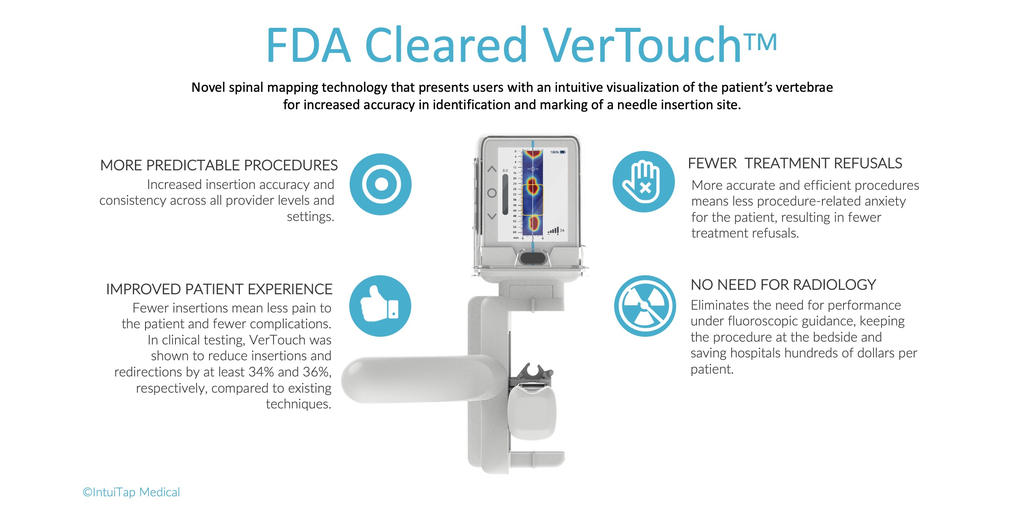
IntuiTap’s VerTouch device is a handheld imaging tool designed to assist healthcare providers of all levels of training and experience to consistently and accurately perform spinal punctures at the bedside. Before this advance, the standard of care has been for healthcare providers to feel the patient’s vertebrae by hand and blindly insert the needle. Unsurprisingly, this method can be both unpredictable and unreliable which results in first-attempt failures in 40-60% of cases. That outcome is at worst a traumatizing and at best less than ideal patient experience. It also leaves room for complications that can result in significant cost to the healthcare system. Over 47% of patients requiring a spinal tap in emergency departments today are sent to radiology, costing hospitals and patients millions each year in additional testing.
Leveraging IntuiTap’s proprietary spinal mapping technology, VerTouch now enables providers to generate a 2D image of lumbar spinal anatomy, helping them visualize the key landmarks of the patient’s spine to inform their decision and guide where to initiate needle placement.
By enabling greater procedure accuracy and efficiency, VerTouch has the ability to improve patient outcomes, reduce procedure related anxiety and treatment refusals, and reduce referrals to radiology, significantly reducing healthcare costs.
“Having spent more than two decades pioneering the use of ultrasound to improve emergency lumbar punctures, I know the challenge of these procedures and that difficult training and image interpretation prevent ultrasound from being a complete solution,” said Dr. Michael Blaivas, MD MBA, Co-Founder and Past President, Society of Ultrasound in Medical Education. “I am thrilled about the emergence of VerTouch as an intuitive, efficient technology to finally address the limitations of traditional and ultrasound-based procedures.”
“Receiving FDA clearance for our novel VerTouch device marks a crucial milestone in our team’s journey to making epidurals, spinals, and lumbar punctures more accurate and efficient,” said co-founder and Chief Executive Officer of IntuiTap Medical, Jessica Traver. “We are proud to have developed a device that can improve procedure experience and outcomes for providers and patients alike, while also saving healthcare systems the cost of failed attempts. We cannot wait to begin launching VerTouch in hospitals across the country within the year.”
IntuiTap Medical is considering global partnerships as they prepare for their own launch. VerTouch is expected to be available to patients within the year.
About IntuiTap Medical
IntuiTap Medical is a medical technology company committed to changing the current state of the art for epidurals, spinal taps and lumbar punctures through their revolutionary, FDA cleared medical device VerTouch IntuiTap Medical is based in Houston, TX. For more information please visit their website at intuitapmedical.com.
About VerTouch
VerTouch is an FDA cleared medical device that utilizes proprietary spinal mapping technology to improve the accuracy, efficiency and predictability of patient and healthcare provider outcomes for epidural, spinal and lumbar puncture procedures. More than 12.7 million of these procedures are performed annually in the United States.
Contacts
Jessica Traver
Chief Executive Officer, IntuiTap Medical
info@intuitapmedical.com